Specialty in Europe
European Affairs

The UEMS house is located in the heart of Brussels EU quarter – a stone’s throw from the Parliament and the Commission – and the UEMS is naturally very active in political lobbying with EU Institutions, addressing the main challenges faced by European health sector, including standardisation of training, accreditation and practice.
The European Union of Medical Specialists has indeed a long-term tradition in defending the interests of medical specialists, as it first convinced the European Commission that health care required a Directive establishing comparable high levels of medical training in the Member States. Further examples of EU-related policy statements are the UEMS Declarations on Quality (Trilogy): Policy on Continuing Professional Development (2001), Promoting Good Medical Care (2004), Ensuring the Quality of Medical Care (2007).
The UEMS is now working in close consultation with other European Medical Organisations (AEMH, CEOM, CPME, FEMS, EANA, PWG, UEMO) and many other scientific Non-Governmental organisations and patient associations.
The Section of Radiology itself is deeply monitoring the European standards in Medical Training, competence-based training and assessment, Professional Qualifications directives, Cancer Prevention initiatives, Personalised Medicine & eHealth and the most relevant thematic networks and public consultations on the EC Health Policy Platform. In addition, the Section is actively engaged in the fields of education and post-graduate specialist training through the collaboration with the European Society of Radiology, the European Board of Radiology and the most important scientific societies.
To follow-up the most relevant EU and International dossiers, the Section actively collaborates with the European & International Affairs dept. of the European Society of Radiology, which offered to join forces with the UEMS on topics of mutual interest, such as:
Education & Training Professional Qualifications Directive Value-based Healthcare Quality & Safety, Radiation Protection EU Cancer policies Research Artificial Intelligence eHealth Medical Devices

The impact of Brexit on Radiology
A referendum was held on 23 June 2016 to decide whether the United Kingdom should leave or remain in the EU. Leave won by 51.9% to 48.1%. For the United Kingdom to leave the EU, it had to invoke Article 50 of the Lisbon Treaty, which gives the two sides two years to agree the terms of the split. Prime Minister Theresa May triggered this process on 29 March 2017, meaning the United Kingdom is scheduled to leave at 11 p.m. U.K. time on Friday, 29 March 2019. After a negotiation process, the United Kingdom finally left the EU at the end of 31 December 2020.
Professional consequences after post-Brexit vote
The chronic shortage of radiologists in UK National Health System is becoming dramatic following the Brexit vote in June, Royal College of Radiologists (RCR) President Dr. Nicola Strickland said in several interviews last month – and the situation is getting worse. A third of UK radiologists already come from abroad and new recruitment campaigns have been launched in the past 6 months, without success. Fears generated by Brexit hurricane and potential visa restrictions led overseas consultants not to fill the vacancies, as uncertainty on British politics would made impossible to relocate with their families to the British Isles. Dr. Strickland added Health Secretary Jeremy Hunt’s proposal to train an additional 1,500 doctors each year will not possibly solve the problem, as only a small number of them will choose the radiology specialty and they won’t even come through the system until 2030.
According to a census released by the Royal College of Radiologists (RCR) on 10 September 2016, demand for imaging services increases, but the supply of radiologists does not, if we just consider that between 2012 and 2015, the consultant radiologist workforce in Scotland grew by 5%, but the number of CT and MRI scans rose by 55%. A key finding is that 99% of U.K. radiology departments could not already meet scan and x-ray reporting demands. Severe diseases as aggressive cancers may go undiagnosed and life-saving treatments become unavailable because of the shortage, while patients would be forced to seek for urgent treatment abroad.
After Prime Minister Theresa May´s decision to support a full and radical break from the EU, the British National Health System – NHS and the Royal College of Radiologists find themselves in a dramatic situation: while the Government has made clear it wants to allow current EU immigrants, a significant part of the the workforce, to remain after Brexit, almost nothing has been done in the last months to combat the increasing shortfall in radiologists, warns the President of the Royal College of Radiologists – Dr. Nicola Strickland. With controls on qualified immigration, recruiting a new generation of radiologists is becoming more and more difficult – Dr. Strickland added – and reliance on teleradiology could be the only solution. Among many other consequences, the European Medicines Agency (EMA) will probably relocate from its current headquarters in London, meaning Britain will lose a prestigious institution in the healthcare field and the power of influencing health policies. Furthermore, UK policymakers still need to decide if to secure a deal with EFTA and how to cope with the European Committee for Standardisation after rejecting the European Union and the related European Economic Area agreements. A complicated transition period in import-export of CE marks and standards, with a bad impact on imaging hardware and IT products in the UK market.
Radioisotope supply has been another significant concern with Brexit, especially with a planned move for the U.K. to leave the EU Euratom regulatory body in favor of its own Office of Nuclear Regulation.While facing Brexit negotiates, the Royal College of Radiology highlighted how most materials for PET/CT scanning are manufactured in the U.K., but the rest of the country’s radioisotope supply is still imported from Europe and other regions, thus creating a serious risk of future shortages if the UK leaves the Euratom treaty under Brexit.
In February 2018, Brexit negotiations were still going on in Brussels, while the health topics risk being forgotten, potentially delaying the availability of new medicines and imposing large costs on patients, hospitals and manufacturers. Healthcare is usually among the most important policy issues to voters at national elections but has largely been absent from the recent Article 50 talks between the EU and UK. That is despite the fact that the UK pharmaceutical industry is one of the country’s largest, with approximately €3 billion of medical devices imported to the UK from the EU-27, compared to exports of €2 billion. Pharma industry leaders have already warned that they face multi-million euro contingency costs, while some clinical trials on new medicines will be put on hold until they have greater certainty on what the UK’s relationship with the EU is likely to look like. Pharmaceutical companies face a multi-million euro bill in regulatory costs regardless of whether the UK reaches a ‘soft’ or ‘hard’ Brexit deal with the European Union.
The agreement on Phase One of the Brexit talks struck in December 2017 protects patients who have already started a treatment when the UK leaves the EU in March 2019, although a ‘hard Brexit’ would pose the greatest disruption for the European healthcare sector and patients, particularly if there is no mutual recognition agreement on clinical trials, batch testing and diagnostics. Patients could face a long delay for new drugs even if the UK swiftly agrees on a free trade deal with the EU, according to the Brexit Health Alliance, similarly to what happens in Switzerland, which starts receiving new drugs an estimated 157 days after the EU, despite having a series of bilateral trade agreements with it, also because drug companies tend to target their new products at the largest markets. Meanwhile, since Britons voted for Brexit in June 2016, the National Health Service – the country’s largest single employer – has been concerned that the health service, which is already facing a staffing shortfall, could be deprived of thousands of EU nationals after the UK leaves the single market at the end of 2020.
Nationals from other EU countries make up almost 10% of doctors in England’s hospital and community health services, and just over 7% of all nurses. However, last available data does not yet suggest that an exodus of EU health workers from the UK has taken place. Around 62,000 known EU nationals work in the NHS, accounting for 5.6% of the total workforce, a slight increase from 5.5% in early 2016. While it is still unclear whether the UK will continue to participate after Brexit in the European Reference Networks, on February 28, 2018 the Brexit Health Alliance, which consists of organisations from the NHS, medical research, industry, patients and public health organisations, warned about the consequences that a “bad Brexit” deal could have on patients with rare diseases. The report remarked the importance of the European Reference Networks (ERN), whose aim is to provide cross-border healthcare cooperation between European countries on treating rare and complex diseases. The European Commission has insisted that the ERNs are significant because almost 30 million patients across Europe suffer from rare diseases.
An interview with Dr. Nicola Strickland, President of the Royal College of Radiologists (RCR), on Brexit from ECR 2019:
https://www.auntminnieeurope.com/index.aspx?sec=rca&sub=ecr_2019&pag=dis&ItemID=617048
Healthcare in Europe
European Semester
The European Semester provides a framework for the coordination of economic policies across the European Union: introduced in 2010, it ensures that Member States discuss their economic and budgetary plans with their EU partners at specific times in the first part of the year – hence the term Semester – so that national action can be accordingly taken in the second part of the year, notably with the adoption of the budgets for the subsequent year.
However, as a concrete response to the coronavirus pandemic, the European Semester 2021 timeline and goals will be adadpted according to the Recovery and Resilience Facility that will make € 672.5 billion in loans and grants available to support reforms and investments undertaken by Member States. The aim is to mitigate the economic and social impact of the coronavirus pandemic and make European economies and societies more sustainable, resilient and better prepared for the challenges and opportunities of the green and digital transitions.
EMSA statement on Conflicts of Interest in Medical Education Settings
The European Medical Students’ Association (EMSA) recently adopted the policy paper “Conflicts of Interest in Medical Education Settings” at the General Assembly in Heidelberg on 26/04/2019. Awareness of conflicts of interest in healthcare collaborations and education on how to handle them are essential for the professional and scientific integrity of future doctors. This is being widely neglected in European medical faculties. Therefore, EMSA calls on European medical faculties to: firstly, implement courses on how to deal with conflicts of interest and secondly, adopt a guideline regulating their own handling of conflicts of interest. Furthermore, EMSA calls on European Institutions and member states to raise public awareness for this topic, encourage medical schools to implement courses on the topic and allocate financial resources on training and research regarding conflicts of interest in healthcare. EMSA acknowledges the importance of handling conflicts of interest as part of medical education and commits itself to work on it.
Link to the statement:
EMOs Statement on Prevention of communicable diseases through vaccination
European medical organisations reaffirm that the prevention of communicable diseases through vaccination is safe and very effective. Immunisation through vaccination is the best protection we have against serious, even deadly, diseases.
In recent years, vaccination coverage for children and adults has decreased in many European countries. Vaccine hesitancy has become one of the main challenges and reasons for low vaccine acceptance and uptake. The influence of diverse anti-vaccine groups spreading misleading and often false information has increased, especially on social media. In Poland, a draft law amending the legislation on communicable diseases has been prepared against medical advice, submitted to the Parliament as a citizens’ initiative and rejected only after a decisive protest from medical organisations, experts and public. This example shows that the risk of abolishing the obligation of preventive vaccinations and in due time exposure of many citizens to dangers of serious contagious diseases that are currently controlled by national vaccination programmes is real.
We believe that existing legislation in European countries should in no case be amended to weaken the role and scope of national vaccination programmes. Coordination and cooperation between different European countries should be strengthened to prevent communicable diseases that know no borders. Also, doctors, other healthcare professionals and their organisations are essential to deliver facts based on scientific evidence and increase public awareness about the benefits of immunisation. Our voice based on our professional knowledge and expertise should be listened to, and the facts based on sound science should be respected. European medical organisations call on governments and legislators to assure that vaccination programmes are supported in the best interest of European citizens.
—————
European Association of Senior Hospital Doctors(AEMH) European Council of Medical Orders(CEOM) Standing Committee of European Doctors(CPME) European Working Group of Practitioners and Specialists in Free Practice(EANA) European Junior Doctors Association(EJDA) European Medical Students Association(EMSA) European Federation of Salaried Doctors(FEMS) European Union of General Practitioners(UEMO) European Union of Medical Specialists(UEMS)
ECHI – European Core Health Indicators
The European Core Health Indicators (ECHI), formerly known as European Community Health Indicators, is the result of long-term cooperation between EU countries and the European Commission. Three ECHI projects (1998-2001, 2001-2004, 2005-2008) funded under the EU Health Programmes established the first lists of ECHI indicators, aiming to provide comparable health information and knowledge system to monitor health at EU level. ECHI indicators are grouped under five main chapters: Socio-economic situation, health status, health determinants, health services and health promotion.
A relevant example of health services is the distribution of Computer Tomography scanners and Magnetic Resonance Imaging units per 100.000 inhabitants. Indicators are at the crossroads of policy questions and data sets. They reflect a policy interest as well as a selected set of possibilities in terms of what can be calculated. Medical Technologies (e.g. CT scanners and MR imaging units have been included in ECHI statistics: Medical technologies: MRI units and CT scanners (I)
Facts & Figures in Europe
European Quality of Life Survey
Nearly 37,000 people in 33 European countries (28 EU Member States and 5 candidate countries) have been interviewed in the last quarter of 2016 for the fourth wave of the European Quality of Life Survey. This overview report presents the findings for the EU Member States. It uses information from previous survey rounds, as well as other research, to look at trends in quality of life against a background of the changing social and economic profile of European societies. Ten years after the global economic crisis, it examines well-being and quality of life broadly, to include quality of society and public services. Each Member State exhibits certain strengths in particular aspects of well-being, but multiple disadvantages are still more pronounced in some societies than in others, in relation ; and in all countries significant social inequalities persist.
Download the report at the link below: https://www.eurofound.europa.eu/publications/report/2017/fourth-european-quality-of-life-survey-overview-report
On 21-22 June 2018 the new Joint Action Health Equity in Europe was launched in Luxembourg in the presence of the EU Commissioner Andriukaitis. Forty-nine participants from 25 EU Member States work together to address health inequalities and underlying social determinants of health across Europe. Under the coordination of the Italian Institute of Public Health, the Joint Action aims to achieve greater equity in health in Europe across all social groups while reducing the inter-country heterogeneity in tackling health inequalities.
European Observatory on Health Systems and Policies
The European Observatory on Health Systems and Policies supports and promotes evidence-based health policy-making through comprehensive and rigorous analysis of the dynamics of health care systems in Europe. The Observatory is a partnership that includes the Governments of Austria, Belgium, Finland, Ireland, Norway, Slovenia, Sweden, Switzeland and the United Kingdom; the Veneto Region of Italy; the French National Union of Health Insurance Funds (UNCAM); the World Health Organization; the European Commission; the World Bank; the London School of Economics and Political Science (LSE; and the London School of Hygiene & Tropical Medicine (LSHTM). The partnership is hosted by the WHO Regional Office for Europe.
For further information: http://www.euro.who.int/en/about-us/partners/observatory/about-us
EU Health Policy Platform
The Health Policy Platform (HPP) has been presented in Brussels on December 05, 2016. More than 150 health interest groups already registered, an Agora space, 4 Thematic Networks (more to come), and 23 Networks of EC experts and stakeholder groups. HPP is used by other international organisations (e.g.: Steering Group of the WHO European Health Information Initiative). Registered users receive notifications about newly uploaded information. Moreover, DG SANTE has created an online resource centre in the Agora Library of the EU Health Policy Platform with a range of material on new care models and integrated care. The purpose is to offer a structured reference area – a “hub” – where care authorities and other interested stakeholders can find readily available knowledge and tools, to help them build their know-how and capacity to implement integrated care and new care models. This document has collected relevant material, grouped under the following categories: 1. Good Practices 2. Case Studies 3. Tools 4. Guidance for Designing and Implementing Integrated Care 5. Assessing Integrated Care 6. Contracting and Payment models for New Care Models 7. Financing Instruments and Mechanisms.
You can register to the Platform here: https://webgate.ec.europa.eu/hpf/
EU Expert Panels
1) The Expert Panel on effective ways of investing in health is an interdisciplinary and independent group established by the European Commission which could support the EU countries in delivering high quality care and making their health systems more effective, accessible and resilient. The Panel has adopted three opinions related to access to healthcare, innovative payment models for high-cost innovative medicines and performance of primary care.
- The Opinion on innovative payment models for high-cost innovative medicines analyses how national pricing and reimbursement authorities could improve patients’ access to innovative medicines and foster innovation that matters whilst ensuring that health systems are financially sustainable. The Opinion sets out some broad principles to guide the definition of specific payment models, including greater price and cost transparency, looking at patent law and market exclusivity rules to promote and reward high-value innovations, and using methods to measure the social value of pharmaceutical products, e.g. in the context of Health Technology Assessment (HTA).
- The Opinion on benchmarking access to healthcare in the EU responds to the request for quantitative and qualitative benchmarks to assess progress in reducing unmet need for healthcare. It is based on the data collected through the EU annual Survey of Income and Living Conditions (EU-SILC), drawing attention to evidence of relatively high rates of unmet healthcare needs in some EU countries. The Panel advised on how to identify the distribution of unmet needs and how to address challenges by mobilising resources at national and European level.
- Finally, the Opinion on tools and methodologies for assessing the performance of primary care explores how to measure the performance of primary care. It takes account of the complex structure, modes of operating and services provision of primary care, and also of its outcomes, particularly in terms of relevance, equity, quality and financial sustainability. The Opinion translates multiple dimensions into comparative key indicators, and descriptive additional indicators, related to the 10 identified domains of primary care.
More information available on the EU Health Policy Platform: https://webgate.ec.europa.eu/hpf/item/item/8493
2) The European Commission’s Scientific Panel for Health (SPH) is a science-led group consisting of 27 experts based on the provisions of the Horizon 2020 Specific Programme that has been tasked with helping to achieve better health and wellbeing for all.
European Reference Networks (ERN)
Approximately 30 million European citizens are affected by rare diseases. They face major challenges in diagnosis, treatment and care of their rare and complex conditions.
European Reference Networks (ERNs) are virtual networks involving healthcare providers across Europe that use a bespoke IT platform and telemedicine tools to review patient cases; they aim to tackle complex or rare diseases and conditions that require highly specialised treatment and concentrated knowledge and resources. Where possible, they ensure that it is the information that travels, not the patient.
With ERNs, patients with rare and complex conditions will be able to benefit from the best treatment and advice available in the EU for their specific condition. Their doctors will have access to a highly specialised pool of colleagues from all over Europe.
The driving forces behind the ERNs are healthcare providers and national health authorities. They show trust, take ownership and have the most active role in the development and functioning of the networks. The Commission’s role, as defined in the 2011 EU Directive on Patients’ Rights in Crossborder Healthcare, is to create the framework for the ERNs. The Commission also provides grants to support network coordinators and provides them with the technical networking facilities. The 4th ERN Conference (Brussels, November 21-22, 2018) opened a new stage in the networks’ lifecycle, namely the deployment phase. After an intensive period of preparatory actions and awareness raising , the first 24 ERNs are operational.
Health Technology Assessment (HTA)
Health Technology Assessment (HTA) refers to the the systematic evaluation of properties, effects, and/or impacts of health technology. It is a multidisciplinary process to evaluate the social, economic, organizational and ethical issues of a health intervention or health technology. The main purpose of conducting an assessment is to inform a policy decision making.
HTA is a research-based tool to support decision-making in healthcare. HTA assesses the added value of new health technologies over existing ones, including medical devices anjd diagnostic tools. HTA is used with a view to improving the quality and efficiency of public health interventions and the sustainability of the entire healthcare system, involving both national and regional players. The European Commission has recently published an inception impact assessment for an initiative on HTA, planned for the fourth quarter of 2017 which will be preceded by a public stakeholder consultation due to be launched in late autumn 2016.
On 22 September 2016, DG SANTE met with EUCOMED and EDMA representatives, both members of MedTech Europe alliance in relation to the recent publication of the DG SANTE Inception Impact Assessment (IIA) on strengthening the EU cooperation on HTA (14 September). Representatives of the European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry, COCIR were also present as observers. The morning session, hosted by EUCOMED, focused on medical devices while the afternoon session, focused on in vitro diagnostics, was introduced by EDMA. The objective of the meeting was primarily for the European Commission to present the initiative strengthening the EU cooperation on HTA and to explain the next steps including the imminent public consultation and recently launched studies; and for the medical technologies sectors to provide initial comments on the initiative.
The European Network for Health Technology Assessment currently focuses on a core model for HTA, consisting of nine domains:
- definition of the health problem and current use of technology;
- description and technical characteristics of the technology;
- issues of safety; issues of clinical effectiveness; cost and economic evaluation;
- ethical aspects; organisational aspects; patient and social aspects; and legal aspects.
The Commission has received some 250 replies to its public consultation on strengthening EU cooperation on Health Technology Assessment, 25% of which from citizens and 75% from stakeholders. Almost all respondents acknowledge the usefulness of HTA and 87% consider that EU cooperation on HTA should continue beyond 2020. Of those who support sustainable EU cooperation on HTA, many think the scope should include pharmaceuticals and consider that medical technologies should also be covered.
The European Commission’s upcoming proposal on Health Technology Assessment which measure the added value of a medical equipment or device compared to existing ones – will focus on clinical aspects and leave member states free to decide on economic or ethical issues. Therefore, the Commission intends to reassure worried member states and aims at addressing the issue of having multiple national assessments for the same products, with subsequent lack of coordination. In the last years, the challenge posed to national healthcare budgets by the high prices of medicines and medical devices triggered a lot of political discussions and menaced the political values of equity of access and innovation. It is then in the common interest of all citizens that Member States rely more on each other, share information and have more tools at their disposal to help them take informed decisions when assessing the added value of innovative medicines, procedures and equipments.
A legislative proposal on strengthening the EU cooperation on HTA while assuring the principles of proportionality and subsidiarity is scheduled to be adopted by Commissioners on 31 January. HTA is a multidisciplinary process to assess the added value and effectiveness of a given health technology – for example medicine, medical devices, diagnostic tools or surgical procedures, over and above existing ones. The Commission hopes that HTA will be used by member states as a tool to ensure cost-effective, accessible and – most of all – sustainable health systems.
The scope and competences of an EU HTA have divided the member states with the countries that already have an HTA preferring a less centralised role while others wanting a strong mechanism. The EU presidency believes it is essential that the member states have sufficient time at their disposal to analyse the text, which will apparently require coordination between various authorities in each country. The Council recognised that EU cooperation on HTA can support the decision-making of Member States and asked the Commission to reflect about the future of this cooperation beyond 2020 when the current EUnetHTA Joint Action comes to an end. The European Parliament in its Report on EU options for improving access to medicines highlighted the potential of joint assessments for avoiding the duplication of efforts and the misallocation of resources across the EU and urged the Commission to propose legislation on a European system for HTA.
More information available at: http://ec.europa.eu/research/health/index.cfm
Digital Health
The introduction of digital technology in EU national healthcare systems is now reality: the spread of ICT tools have finally enabled a new frontier in big-data storage that will have a tremendous impact on the national healthcare systems. For that reason, the new EC report on Big Data & Healthcare recommends the creation of a unified platform (EHRO – Electronic Health Records Organisation) where patients and physicians can access, modify and (eventually) delete data, including sensitive information. The creation of the EHRO aims at overcoming past challenges faced by member states as the collection of patients’ data, its ownership, and processing, including privacy issues. By sharing personal health information on EU level, patients wishing to receive treatments abroad, will still be able to receive appropriate care and avoid unnecessary medical fees and administrative burdens (e.g. avoidance of duplicate medical tests). On the other hand, practitioners will have a comprehensive view of the patient’s medical background, enabling them to offer the most appropriate and rapid treatment. If individuals will be given the chance to give an informed consent to share their data, this will result in a less problematic approach than making use of data extracted from other sources (e.g. social media, surveys…): eHealth stakeholders, however, should continue to carefully consider ePrivacy and security issues when it comes to discuss their future strategies.
On 12th May 2017, the European Commission has published the mid-term review of the Digital Single Market (DSM) Strategy. Adopted in 2015, the DSM aims to make the EU’s single market freedoms “go digital” and boost growth and jobs in the EU.
- The percentage of hospitals exchanging patient information electronically with other health care organisations within the same country ranges from 33% to 39%. Exchange with health and care providers in another EU country is only 4%.
- 52% of citizens wish to have electronic access to their health records, but only 9% of hospitals in Europe allow citizens to access online their own patient records, even partially.
- Faster diagnosis and more personalised treatment of rare and complex diseases can be achieved if scientific expertise and data are pooled across borders, significantly reducing the 5.6 years on average that it takes currently to diagnose a rare disease in Europe. An estimated 30-40 million Europeans are affected by rare and complex diseases.
- Faster diagnosis of infectious diseases and more effective response can be achieved if scientific expertise and health data are pooled across borders.
- Europe has the highest burden of chronic diseases which are responsible for 86% of all deaths and 77% of health and long-term care expenditure. But only 18% of European citizens have used online health and care services in the last 12 months.
- By improving interaction between users and health care providers, mhealth can improve quality of services and better planning by healthcare systems. If widely adopted, annual savings in Europe resulting from use of mhealth applications are estimated at € 69 billion.
The White Paper on the Future of Europe – officially presented at the last eHealth week in Malta – notes that by 2030 Europe will be the oldest region in the world. Expenditure on health and long-term care has been increasing in all EU Member States, and is expected to rise even further as a consequence of an ageing population. Despite an increased spending in health and care, there has been a decline in the average healthy life years of citizens across the EU28. Therefore, enabling a better use of the digital technologies may improve the citizens’ health, and address the systemic challenges which our healthcare systems are facing.
mHealth
mHealth is referred to as “medical and public health practices supported by mobile devices”. Mobile devices can now be used to monitor, record, analyse, alerts and communicate health information to reach people and professionals remotely. It may deliver behavioural interventions to support individuals to start, reinforce or reduce specific health behaviours. mHealth has the potential to increase accessibility and to contribute to a more person-focused healthcare system, support shifts towards prevention, and improve system efficiency. It could also contribute to making access to healthcare more equitable.
Although the annual growth rate of the European general radiography market has stalled to a near standstill, mobile general radiography systems can be used in a wider variety of clinical settings, including emergency departments, intensive care units, and operating rooms, improving workflow efficiency and patient access to diagnostic imaging. More diversity in the design of mobile digital systems, particularly lightweight and compact systems, coupled with extended battery life, is increasing their versatility for bedside imaging. Hospitals have typically set as a top priority the digitialization of fixed rooms and the replacement , and there is still a sizeable stock of analog mobile systems in use. These will gradually be replaced with digital systems over the coming years. The cost for a digital replacement can typically be justified by the operational efficiency gains and reduced dose associated with digital mobile systems.
With an almost flat growth forecast for the general radiography market in Western Europe, the best opportunities for growth are now located in the East. The Eastern European DR market is – however – heavily influenced by the availability of EU development funds for healthcare projects and the recent announcements that EU funding is to be shifted away from Central and Eastern Europe to the countries worse hit by the financial crisis, (e.g. Spain and Greece), cast a shadow of doubt over the longer-term growth prospects for the region.
Link: http://eurohealthnet.eu/publication/making-link-mobile-health-mhealth
IC Health – Improving Digital Health Literacy in Europe
Citizens’ digital health literacy is now considered an essential element for successful eHealth deployment in Europe. However, citizens often do not have the necessary skills to find and evaluate online health information and apply their knowledge to make health decisions. Digitally health literate citizens are empowered to play a more active role in their health self management, resulting in improved prevention, adherence to a healthier lifestyle and better health outcomes.
IC-Health project will provide support for the improvement of digital health literacy in Europe; in particular, the project will design 35 open access online courses (MOOCs), in eight different national languages, for different population cohorts including for instance children, pregnant awomen, elderly and people affected by diabetes. The Platform is composed of different “rooms”, each one dedicated to a specific community of practice, divided per country. In addition, there is an extra room called ‘Europe’ devoted to the exchange of knowledge and information on digital health literacy at Eu level and to support the creation of a wider network of interest around the work of the IC-Health project.
To register, please visit: http://ichealthplatform.eu
Personalised Medicine
The Horizon 2020 Advisory Group has defined personalised medicine as “a medical model using characterization of individuals’ phenotypes and genotypes (e.g. molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention“.
Personalised medicine does not only concern medicines or medicinal products. A better understanding of the biological mechanisms and environmental interactions that govern health and disease will impact the entire health care continuum, from health research to patient care.
The Directorate-General for Research and Innovation has released the Personalised Medicine Conference Report after the conference which took place in Brussels on June 1-2, 2016. This report addresses the broader policy perspective and challenges by showcasing both integrated healthcare models in Member States and business approaches which involve patients more directly in their healthcare. Attached, the Council´s Conclusions on Personalised Medicine for patients. Please find the full report in the attachments below. An International Consortium for Personalised Medicine (ICPerMed) was initiated during several workshops organised by the European Commission throughout 2016. ICPerMed aims to provide a flexible framework for cooperation between its member organisations.
For detailed information on the Personalised Medicine Agenda in the EU, please visit:
https://ec.europa.eu/research/health/index.cfm?pg=policy&policyname=personalised
In 2013, the European Commission Health & Food Safety Directorate-General (DG SANTE) published a staff working document (SWD) on the potential use of ‘–omics’ technologies in the research and development of personalised medicine, on recent developments in EU legislation concerning medical devices and on socioeconomic factors affecting the development of personalised medicine. The ESR – European Society of Radiology has released an ad-hoc statement on the topic.
Medical Devices
On May 25, 2016, the Dutch Presidency of the Council of the European Union and the European Parliament have reached a draft agreement on the medical devices and in vitro diagnostic medical devices regulations. This agreement comes after a four-year long legislative process initiated by the European Commission’s 2012 Proposal for a Regulation on medical devices and Proposal for a Regulation on in vitro diagnostic medical devices to replace the three existing medical devices directives. The Commission had considered a revision of the existing regulatory framework necessary to enhance the safety of medical devices while allowing patients to benefit from harmonised rules for timely and innovative health care solutions. Devices will in future become fully traceable and patients receiving implantable devices must be informed of the product’s key facts. Non-confidential information will be made available publicly through the central database European Databank on Medical Devices (Eudamed). Another consequence will be the adoption of tighter rules for the notified bodies that are responsible for assessing medical devices, ensuring they have available qualified personnel to conduct factory inspections. The new regulation will also hold manufacturers responsible for continuous follow-up on the quality and safety of devices placed on the market, mandating manufacturers to monitor and act promptly in case emergencies arise.
Among the reactions, Serge Bernasconi, CEO of MedTech stresses the importance of collecting necessary funds from public and private stakeholders (e.g. Governmental Health Agencies, Industry Representations) to fully implement the agreement, and confirms that industry bodies EDMA and Eucomed will actively support their members in the challenging transition towards a new, less bureaucratic regulation. The European Patients’ Forum (EPF) would have expected a closer involvement of patients, but still welcomes the agreement as a means to implement stricter controls on pre-market assessment and post-market surveillance of medical devices, resulting in better safety standards for patients.
The May 25 agreement still needs to be formally adopted by the Council and the Parliament to conclude the legislative process. It is also important to note the transition periods for the new rules, which will apply three years after publication of the medical devices regulation and five years after publication of the vitro diagnostic medical devices regulation. On April 5, 2017, the European Parliament finally adopted two new regulations imposing stricter rules on medical devices’ safety, which, in any case, will not enter into force before mid-2020. The regulations will be fully applicable in three years for medical devices and in five years for in vitro diagnostics.
The two new Regulations bring a number of improvements for medical and in-vitro devices:
- Improve the quality, safety and reliability of medical devices: The new rules will impose tighter controls on high-risk devices such as implants, requiring a pool of experts to be consulted before placing the device on the market. Controls will also be tightened on clinical trials as well as on the bodies that can approve the marketing of medical devices. Medical devices such as breast or hip implants will be traceable and the post-market surveillance will be reinforced. In addition, the regulation will provide random inspections of producers’ facilities after devices have been placed on the market, as well as stricter controls on notified bodies, which will have to employ medically skilled people.
- Strengthen transparency: The new regulations will make sure that vital information is easy to find for consumers. For instance, patients will receive an implant card with all the essential information, and a unique device identifier will be mandatory for every product so that it can be found in the new European database of medical devices (EUDAMED).
- Enhance market surveillance: Once devices are available for use on the market, manufacturers will be obliged to collect data about their performance and EU countries will coordinate more closely in the field of market surveillance.
EPF statement for swift and effective implementation of the new EU Regulations on Medical Devices to improve safety and transparency
Patients with chronic conditions rely on medical devices to improve their health and quality of life. Patients have a fundamental right to expect that the devices they use are safe, after being authorised for use in the EU.
The European Patients’ Forum (EPF), an umbrella organisation of 72 patient organisations across the EU calls on the EU Member States, the European Commission and the industry to ensure a swift and effective implementation of the new, improved regulatory framework in a way that will ensure patients’ safety and a transparent regulatory framework. “The system should be fully transparent, with publication of all evidence on safety and quality, as well as public reporting of incidents for devices just as adverse drug reactions are reported”, says EPF’s Secretary General, Nicola Bedlington. “This is vital to restore the trust and confidence of patients and the public in the way medical devices are regulated in the EU”, she added.
As medical devices are produced and circulate all over the European Union, it is important to have common rules to ensure devices on the European market are safe and high quality. In April 2017, following several years of discussions, the EU adopted two new Regulations on medical devices, which replace the existing Directives. The new rules will apply from spring 2020 for medical devices and from spring 2022 forin vitro diagnostic devices.
Stronger safety requirements
The new Regulations will bring important changes for patients, one of which is stronger safety requirements. There are three stages which are crucial for patient safety: clinical investigation, when the device is tested; conformity assessment, when the safety and performance of the device is assessed; and vigilance, which is the ongoing monitoring of risks and incidents once the device is on the market. In our view patients should be empowered to play a role in each of these steps. In particular, patients’ expertise and experiences as users of medical devices needs to be used more effectively in safety monitoring – the long experience with pharmacovigilance has shown that patients’ reports add value and enable any problems to be spotted earlier.
Better information
The new EU Regulations promise better information to patients: an EU database on medical devices, EUDAMED, will be set up and it will be accessible to the public. Patients will be given information leaflets on their implanted devices. An important improvement is that for high-risk, “class III” medical devices, which include implantables, the manufacturer will need to provide a publicly available document summarising the device’s safety and clinical performance. The summary document will be an important source of information for patients and healthcare professionals, and should enable them to make more informed decisions.
The need for stronger controls
Given that a centralised system for approving devices was not going to be an option, EPF called for stronger controls on the notified bodies which approve devices in the EU, to ensure they would not be able to compete by lowering the quality of the assessment procedure and therefore risk patient safety. We believe it is vital that in future the Member States and Commission have control over the notified bodies, and that these are regularly evaluated. The capacity of notified bodies to deliver on the requirements of the Regulation must be ensured.
Patients engage in the implementation process
EPF and our members continue to engage in the implementation process of the EU Regulations. As a member of a sub-group of the Medical Devices Coordination Group, EPF has and will continue to prioritise patients’ safety and call for transparent, easily accessible safety information for patients and their organisations. We have developed materials for patient organisations to inform and enable them to participate in the implementation of the regulations and monitoring of their impact, and continue to work with our community to encourage patient participation at national level.
Patient safety is at the heart of our work for equitable access to high-quality, patient-centred healthcare. Patients across Europe deserve a regulatory system on medical devices that they can trust.
Cancer Prevention in the EU: the state of affairs for 2021
Cancer is still the second leading cause of death in the EU and a major public health concern in terms of disease burden and economic and social cost. There is a constant need for a robust, well informed response to it in order to contribute to the prevention, early detection, and adequate treatment.
While responsibility for the delivery of health services lies with the Member States, the European Union contributes to tackling cancer with awareness-raising, guidance and investment in research and fostering cooperation. In 2009, the Commission adopted the Communication on Action against Cancer: European Partnership and established the European Partnership for Action against Cancer (EPAAC). Building on the EPAAC, the Comprehensive Cancer Control joint action 2014 – 2017 (CANCON) aimed to help reduce the cancer burden in the EU. It delivered a European Guide on Quality Improvement in Cancer Control. An expert group on cancer control has been established by the Commission in 2014, gathering representatives from EU, EEA and EFTA countries, but also patients´ and professional associations and IARC.
Developed in cooperation with WHO, the European Code against Cancer is a set of 12 recommendations as to how people can reduce their cancer risk: it has been constantly updated since 1987. On December 15, 2017 an important anniversary in the fight against cancer has been celebrated in Europe: 30 years ago the first edition of the European Code Against Cancer (ECAC) was published at a time when cancer awareness was almost insignificant. Nowadays, instead, awareness of cancer prevention is on the rise, as – according to a recent online survey by the Association of the European Cancer Leagues (ECL) – 82% of people understand that people can reduce their risk of getting cancer in the future by making changes to their lifestyle.
For more than 30 years since the first ‘Europe against cancer’ programme was launched, actions taken at EU level were implemented to extend and save lives. The Commission has been at the forefront of tackling risk factors, promoting screening to detect cancer early, and best practices to help EU countries improve the quality, effectiveness, resilience of health systems and to reduce inequalities in access to health services. Population-based cancer registries are the data providers that enable the monitoring of cancer frequency and collecting information on new cancer cases in well-defined populations. They are critical resources for the clinical and epidemiological investigation of cancer and for the planning and evaluation of cancer prevention and control programmes.
Europe´s Beating Cancer Plan (February 2021)
On 3 February 2021, the European Commission has presented Europe´s Beating Cancer Plan, a new ambitious approach towards cancer prevention, treatment and care. It will tackle the entire disease pathway (from prevention to quality of life of cancer patients and survivors), focusing on actions where the EU can add the most value and it will be supported by actions spanning across policy areas from employment, education, social policy and equality, through marketing, agriculture, energy, the environment and climate, to transport, cohesion policy, and taxation. It must be reminded Europe’s Beating Cancer Plan is a key pillar of the European Health Union, as presented by President Ursual von der Leyen in November 2020, calling for a more secure, resilient and better-prepared European Union.
The European Society of Radiology (ESR) fully supports the European Commission´s focus on prevention to reduce cancer incidences through increased action on environmental and lifestyle factors as well as medical interventions and welcomes the ambition to improve access to existing screening programmes as well as their quality through the proposed Cancer Screening Scheme: the ESR looks forward to contributing to tools and actions to turn this ambition into reality, particularly welcomes the European Cancer Imaging Initiative to develop an EU ‘atlas’ of cancer-related images and is pleased to see radiation safety is addressed in the Plan.
Link: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_342
European Parliament Special Committee on Beating Cancer
The European Parliament Special Committee on Beating Cancer (BECA) was recently created with the aim to empower the future Europe’s Beating Cancer Plan, reduce the incidence of cancer, help patients live a longer and better life and decrease health inequalities in Europe. The Committee began its work on 23 September 2020 and released a first working document in October 2020.
The European Society of Radiology, representing the voice of European radiologists is pleased to find an emphasis on building upon European initiatives developed by the cancer community and on indicating the current missing data to be addressed in the context of the Europe’s Beating Cancer Plan, the EU Cancer Mission and the EU4Health programme. The ESR is also glad to see areas of action such as patients, prevention, screening and early detection, and the recognition of research and innovation as well as exchanges of knowledge between Member States as possible tools.
More information on BECA composition and work can be found at the link below:
https://www.europarl.europa.eu/committees/en/beca/about
World Health Day (April 07)
World Health Day is a chance to celebrate health and remind world leaders that everyone should be able to access the health care they need, when and where they need it.
Access to healthcare for all is one of the major achievements of post-Second World War Europe bringing to reality the idea that all human beings, rich or poor, should benefit from quality medical care without worry that their illness would bankrupt their family. As we celebrate World Health Day, we should feel a great deal of satisfaction for the progress made on our continent in protecting EU citizens’ health and in raising the overall life expectancy. As a cardiac surgeon, and having treated many patients, I have seen how important Universal Healthcare is, especially for the most vulnerable. I have also met many inspiring healthcare professionals, scientists, researchers and social workers who make Universal Healthcare a reality on everyday basis – it strikes pride in my heart to be part of this wonderful union.
But when it comes to health, an important lesson learned is that borders don’t matter in case of health crisis. That is why the EU has now set up a European Medical Corps to dispatch medical experts to tackle health emergencies such as epidemics or infectious diseasesboth inside and outside Europe. The EU is also a leading global donor for health initiatives to boost research and provide support to fight major diseases across the world. Through its international development funding for example, the EU has allocated over €475 million to the Global Fund to Fight AIDS, Tuberculosis and Malaria. The EU also provides around €200 million in humanitarian assistance every year to support health programmes that seek to limit mortality, disability and disease associated with humanitarian crises. That being said, there are still significant differences in life expectancy and exposure to health risks in the world as well as across the EU. For example, premature mortality rates from chronic diseases are at least twice as high as the EU average in Bulgaria, Hungary and Latvia. Such inequalities are often related to social inequality and caused, in part, by disparities in access to high-quality care due to financial costs and the uneven geographical distribution of doctors within and across EU countries. This is why building a fair and socially equal Europe is a key priority for the European Commission. The European Pillar of Social Rights recently adopted aims to ensure access to social protection for all workers and self-employed individuals in the EU.
This is an important step towards delivering on our commitment to make healthcare accessible to all citizens – not only medical treatment, but also preventive care. Making sure that every single person in the EU has access to health care should be the priority for all national authorities. In this context the continued growth of European Reference Networks will also increase the cooperation across our health systems. These virtual networks facilitate access to specialised healthcare for patients suffering from rare and low prevalence complex diseases.
Although the success of making healthcare available to all ultimately rests with the Member States who are responsible for defining and organising their health policy, services and budget, the Commission will continue to offer guidance in order to decrease the health gaps among EU countries. Together we will be able to make sure that we are doing our utmost so that universal health care is delivered to all, here in the EU and around the world.
Education and Training

European Training Requirements
The UEMS contributed significantly to the improvement of Postgraduate Training through the development of European Curricula in each medical specialties as well as the elaboration of Training Standards. Back in 1994, in fact, the UEMS adopted its “Charter on Training of Medical Specialists” with an aim to outline the guiding principles for high level Medical Training. With five chapters being common to all specialties, this Charter provided a sixth chapter, known as “Chapter 6”, that each Specialist Section was to complete according to the specific needs of their discipline.
https://www.uems.eu/areas-of-expertise/postgraduate-training/european-standards-in-medical-training
This document – renamed European Training Requirements – aims to provide the basic training criteria for each specialty and should be regularly updated by UEMS Specialist Sections and European Boards to reflect scientific and medical progress.
The European Training Requirements for the specialty of Radiology – based on the revised European Training Curriculum prepared by the European Society of Radiology in collaboration with the Section – was originally approved by the UEMS Council in 2013. The curriculum is periodically amended and the last version has been revised in February 2018, endorsed by the Section on October 06, 2018 and by the UEMS Council on October 20, 2018.
This revised version of the Curriculum outlines a five-year training period consisting of Level I (fundamentals) over the first three years, followed by a more flexible Level II with potential special interest rotations during the last two years. Level III is dedicated to the subspecialisation path. Given the developments of the radiology profession, the Section and the ESR request that the name of the discipline be harmonised to “Radiology”. In order to keep pace with the increasing complexity of medical imaging and growing training needs, they also request that the minimum years of training laid down in the European Directives be increased from four to five years.
European Training Curriculum 2018: https://www.myesr.org/education/training-curricula
ETR Reviewing Committee
The ETR Reviewing Committee was established at the UEMS Council meeting in 2013 aiming to support UEMS Specialist Bodies that have produced ETRs to have a comprehensive peer review and possible amendments prior to an open discussion at the Council meeting. Members of UEMS ETR Review Committee are:
- UEMS Secretary General
- two of the UEMS Vice-Presidents
- representatives of the 3 Groupings of the Sections
- three representatives from the NMAs
- administrator from UEMS office
The ETR submitted by the UEMS Sections & Boards for vote and adoption by UEMS Council should follow a precise timeframe:
- first draft to be received by UEMS office no later than 2 months before the Council meeting
- the draft will be distributed to the ETR Review Committee, NMAs and UEMS Sections & Boards for eventual comments
- comments from the above mentioned bodies shall be received no later than 1 month before the Council meeting
- the UEMS body submitting the ETR shall produce a final version of the document no later than 2 weeks before the Council meeting
- the final document will be published 2 weeks before the Council meeting

European Examinations of the Section
The Section of Radiology is particularly engaged in the field of education and postgraduate specialist training.
EDiR – European Diploma in Radiology. Your passport to a better career in Radiology.
The EDiR – European Diploma in Radiology – is an international benchmark for radiologists and is currently a unique and differential advantage over other candidates for a job or fellowship. Created in 2011, it serves the standardisation and accreditation of radiologists across European borders. The examination is open to radiologists and radiology residents in their fifth year of training in compliance with the requirements indicated below.
The EDiR exam consists of multiple response questions, short cases and the Clinically Oriented Reasoning Evaluation. Up to October 2018, 67% of the candidates were ESR Full Members (Europe) and 33% ESR Corresponding Members. Pass rate: 72%.
The application for the EDiR examination can be carried out individually or as a group through hospital heads of department or the corresponding national society.
New: Online e-Examination:
This online EDiR examination will be carried out through dedicated e-Examinations in selected hospitals and learning institutions that meet the venue and technical prerequisites set by the EBR. This is a new online e-Examination format solution:
- It will provide candidates with more flexibility to sit the EDiR examination closer to home.
- For local organisers, it is the safest and most cost-efficient option. The EBR will remotely set up and monitor the examination during the entire process.
With this new format solution, the EBR continues working towards the harmonisation of radiological standards and accreditation of radiologists across European borders through EDiR.
For complete information, please visit the EBR website: https://www.myebr.org/online-e-examination
EBIR – European Board of Interventional Radiology
Activated in 2010, the European Board of Interventional Radiology has obtained UEMS-CESMA recognition in March 2017. Supplemental to any other national qualifications, the EBIR also aims to facilitate the free movement of Interventional Radiologists across and beyond Europe.The EBIR is under the supervision of the CIRSE – Cardiovascular and Interventional Radiological Society of Europe, the ESR – European Society of Radiology – and the UEMS Division of Interventional Radiology. European Board of Interventional Radiology received CESMA appraisal in April 2017.
The 2nd edition of the Curriculum and Syllabus will serve as the basis for the EBIR Examination from March 2018. Preparatory courses for the EBIR are held regularly at the annual scientific meeting of CIRSE, at the European School of Interventional Radiology, the European Conference on Interventional Oncology and through the educational platform ESIRonline.
EBNR examinations
The Division of Neuroradiology collaborates with EBNR to offer EBNR exams in Neuroradiology (EDiNR), in Paediatric Neuroradiology (EDiPNR) & Spine interventional Neuroradiology (EDiSINR). EBNR examinations have received CESMA appraisal in September 2017.
More information at: http://www.ebnr.org
EDiR – European Diploma in Radiology
The EDiR is the European examination offered by EBR – European Board of Radiology and open to radiologists and radiology residents in their fifth year of training in compliance with the requirements. EDiR candidates must be certified radiologists or at least be in their fifth year of national radiology training at the time of the examination. In cases where the radiology training is less than five years, experience as a supervised staff radiologist will be considered. The total of training and supervised practical experience must be four years or more at the time of application.
It has been fully endorsed by the Section of Radiology and has received the appraisal from UEMS-CESMA in 2015. EDiR was recognised as a UEMS examination and examiners involved in EDiR will be granted 2 ECMECS per examination.
UEMS observer in EDiR Scientific Board: H. J. Lamb
UEMS representative in EDiR Standards Committee: M. Adriaensen
The EDiR is recognised as an equivalent of the Polish exit training examination, the first part of the Turkish board examination and the image interpretation part of the Finnish national examination. Moreover, in order to practice radiology in The Netherlands, trainees must either pass their national board examination or the EDiR. It is also recognised in Slovenia and as an equivalent qualification to the Croatian National Board examination in radiology.
It has significant value in many other countries and it is expanding its role beyond Europe. Since 2011, over 3000 candidates have taken the EDiR, with a pass rate of 65.57% in 2019 and 69.05% in 2020.
The EDiR examination has been adapting to the ever-changing circumstances, such as specific national and regional policies issued due to the COVID-19 pandemic. Multiple local examinations with fewer candidates replaced larger EDiR examinations, such as the one that used to take place during the ECR. This new organisational strategy guaranteed access to the examination to those who were affected by travel restrictions.
Structure:
The examination consists of three parts: Multiple Response Questions (MRQs), Short Cases (SCs) and the Clinically Oriented Reasoning Evaluation (CORE). The committees that form the EDiR Scientific Board follow a structured workflow to prepare each examination, ensuring an adequate peer review system for quality assurance.
Mission:
The EDiR reflects the content of the European Training Curriculum (ETC), which covers every aspect of radiology practice. As such, it is an accurate test of general knowledge of the specialty. An ever-increasing number of heads of department are encouraging their final year residents to take the examination. Taking the EDiR is therefore an excellent opportunity for radiologists to test their excellence and compare themselves to their colleagues overseas. Designed to facilitate professional mobility across different countries, the diploma is an asset for radiologists both in and outside Europe. Diploma holders will have an advantage if they are looking for a job. It’s a test of character. It proves that this person wants to compare him or herself to international competition. As Prof. Paul Parizel highlighted: “It proves that this person wants to compare him or herself to international competition.It shows that this person has that kind of energy, it’s more than just an extra line on their CV“.
The Diploma is becoming more and better known and overall more than 3,000 candidates have applied since 2011 (78% have passed), highlighting the solidity of the exam.
How to prepare for EDiR:
The EDiR has toured the world this year, with examinations having taken place in a range of countries including India, Turkey, Spain, Austria, Sweden, France and Egypt. Hundreds have taken the examination, using it as an opportunity to improve their CVs and obtain the certificate of excellence in radiology, which is highly valued across European borders. The EBR is investing in new resources and provided new tools for EDiR candidates, including the EDiR preparatory e-book, the EDiR app, and the EDiR blog.
- The EDiR e-book is intended to be an instrument to help trainees prepare for the EDiR examination. The aim of this book is to provide a standardised study opportunity for radiology residents and facilitate preparation for the exam.
- The EDiR app, a dynamic, interactive tool accessible anywhere and anytime, will contribute to building an EDiR community. It is an attractive, user-friendly application with many assets for up-dating knowledge, including mock examinations with self-assessment tests, MRQs, short cases, structured reports for CORE preparation, notifications, exam examples, etc.
- The EDiR blog serves as a complement to EDiR preparation; in addition to the regular cases that EBR Blog readers are familiar with, it has been recently started a series of ‘Flashcard’ cases. The first two are emergency and neuroradiology cases. Other subspecialties will be successively included to teach candidates and guide them through analysis of useful images. In this way, they will be well prepared for the examination.
- New: EDiR – The Essential Guide, available on: https://link.springer.com/book/10.1007/978-3-030-20066-4
For further information, please contact diploma@myebr.org or visit the EBR website:
CESMA – Council of European Specialist Medical Assessment
“CESMA should become standard setting and accrediting body for all assessments conducted by or with participation of UEMS bodies, and also for other assessments” – Dr. Krajewski and Dr. Borman
CESMA – Council of European Specialist Medical Assessment was created by the UEMS in 2007 with an aim to provide recommendations and advice on the organisation of European examinations for medical specialists:
- To promote harmonisation of European Board assessments
- To provide guidelines to the Boards on the conduct of assessments
- To encourage take up of Board assessments as a quality mark
- To offer an alternative to National assessments, where appropriate
Through the Glasgow declaration in 2007, CESMA set the basis for harmonised standards at the European level with regard to European Specialty Examinations. To the present date, more than 30 European Examinations have been appraised, as marks of excellence in medical specialties. While the European Examinations organised under UEMS aegis are not to be considered as formal qualifications, their quality and renown have increased significantly over the past few years. As a result, some countries recognize European examinations as part of their national examination (more information available in a specific box dedicated to EDiR).
Link: https://www.uems.eu/areas-of-expertise/postgraduate-training/cesma
ETAP 2.0 – Be better. Be certified
The European Training Assessment Programme (ETAP) was established in 2001 as a joint initiative between the EAR (European Association of Radiology) and the UEMS/Radiology Section. In March 2017 it was agreed to shift the project to the EBR. The revised version – European Training Assessment Programme 2.0 (hereafter, ETAP 2.0) – is now an equally represented partnership project between the Section and the European Board of Radiology (EBR). The new ETAP 2.0 has been officially presented at the ESR Annual Leadership Meeting in Barcelona on November 18, 2017 and started its activities at ECR 2018.
The certification process is now performed virtually through the ETAP 2.0 platform, which enables an easy, objective and quick assessment.
Despite the many challenges that the COVID-19 pandemic has caused, ETAP 2.0 has still been able to provide certifications entirely virtually in 2020. The programme has striven to overcome pandemic’s barriers by offering virtual assessment of institutions. As a global programme ETAP 2.0 can provide evaluation of training programmes in any imaging institution in the world, making it easy for any institution to take part in the programme.
Read more about ETAP 2.0 here: https://insightsimaging.springeropen.com/articles/10.1186/s13244-019-0797-4
Goals:
- improve and harmonise the standards of radiology training in Europe.
- provide institutions that offer postgraduate radiology education with objective assessment of their training programmes by external assessors nominated by the ESR.
- develop assessment systems and guidelines for use by postgraduate education authorities at a national level.
The ETAP Working Group, which will become the ETAP 2.0 Scientific Committee after ECR 2018, comprises an equal number of EBR and UEMS members. Candidates from the EBR and the UEMS will take the position of scientific director on an alternating basis. Three EBR members and three UEMS members will also perform the role of assessor on a rotating basis (one lead assessor and co-assessor per assessed institution). A third junior assessor (European Junior Doctors and ESR Radiology Trainees Forum Subcommittee representatives) will perform an advisory role as residents or junior doctors supporting the assessors.
ETAP Scientific Committee:
Scientific Director: H. Aronen
Assessors/ Co-assessors (on a rotating basis):
EBR member: O. Dicle
EBR member: V. Koen
EBR member: L. Oleaga
EBR member: F.B. Pizzini
EBR member: P.K. Prassopoulos
UEMS member: C. Cantwell
UEMS member: I. Battyáni
UEMS member: H. Helmberger
UEMS member: J.P. Joris
UEMS member: J. Kraft
Trainees organisations/advisory role:
P. Largo - former ESR Radiology Trainees Forum Subcommittee M. Pecoraro - ESR Radiology Trainees Forum Subcomittee A. Montvila - European Junior Doctors P. Jylhä-Vuorio - European Junior Doctors
Collaborators/ Verification of information and documentation provided: Society Delegate to the ESR Education Cte. (from the country of the institution to be assessed) and UEMS National Delegate (from the country of the institution to be assessed).

ETAP 2.0 Certification process
Phase 1: Evaluation and completion of the documentation
Centres interested in being assessed submit the application form to the ETAP office using the ETAP platform. The ETAP Scientific Committee verifies compliance with the eligibility criteria and the centre receives its access credentials to the platform to complete the questionnaires. There are two different questionnaires: the main, more extensive questionnaire, to be completed by the head of the training programme, and another one directed to the trainees, to be completed confidentially. Centre and trainees complete their respective questionnaires and provide the necessary information to the assessors.
Phase 3: Online interviews
Online individual, private and confidential interviews are held with the head or the deputy head of the radiology department, head of the education programme, one of the trainees’ tutors, one attending physician or a deputy involved in the training programme, a trainee supervisor, and at least two trainees (one junior and one senior trainee), according to each institutions’ training department structure.
The assessors and co-assessors conduct online interviews and review the questionnaire, the provided documents and videos in order to evaluate the structure and management of the training programme, the delivery of training and education, the radiology facilities and resources, and the outcomes.
A precise analysis of the programme is compiled on a structured feedback form and reported back to the centre. The report analyses strengths, weaknesses, opportunities, and threats (SWOT analysis) and, additionally, provides recommendations to help generate further effective strategies to improve the programme.
Certification levels:
Centres are awarded a certificate according to the assessment performed by the designated assessor and co-assessors. The certificates are valid for a period of 5 years and are subject to renewal. There are three different levels of certification:
- Silver: the institution has training standards that ensure adequate training in accordance with the standards set out in the ETC and covers all aspects of education.
- Gold: the institution provides a standard of training that is in accordance with the ETC, a subspecialisation programme, and basic research training.
- Platinum: the institution provides an advanced subspecialisation and research training programme and all imaging modalities are available.

CME-CPD & accreditation
Continuing Medical Education (CME) consists of educational activities which serve to maintain, develop, or increase the knowledge, skills, and professional performance and relationships that a physician uses to provide services for patients, the public, or the profession. Continuing Professional Development (CPD) is a structured approach to learning to help insure competence to practice, taking in knowledge, skills and practical experiences. It can be defined as the systematic maintenance, improvement and continuous acquisition or reinforcement of the lifelong knowledge, skills and competences of health professionals. (UEMS Glossary of Terms, 2013)
For the UEMS, CME-CPD is:
- a clinical and professional duty
- an ethical obligation
- In the hands of the profession
- Free from commercial influence
- From graduation to retirement
- Meaningful in professional life
- Affordable
- at national and European level
At EU-level, the role of CPD to help safeguard patient safety within the context of cross border mobility has been addressed in several legal instruments: e.g. Council Recommendation on Patient Safety, including the prevention and control of healthcare associated infections, Directive 2011/24/EU on patients’ rights in cross-border healthcare, and Directive 2013/55/EU on the recognition of professional qualifications. National Accreditation Authorities and UEMS Specialty Boards perform professional evaluation of CME-CPD activities through European CME credits (ECMEC). In order to facilitate the transfer of credits between European countries, 1 ECMEC equates to one hour of CME, with a maximum of 6 ECMECs for a day and 3 ECMECs for half a day.
CME-CPD in Europe, an evolving process
A survey from the Section of Radiology
Edited by
Francesco L. Tanzi (Secretariat) and Prof. Paolo Ricci (President of the Section)
The European Union of Medical Specialists (UEMS) is the oldest medical professional organisation in Europe, as it celebrated its 60th anniversary in 2018. With 40 member countries, 60 specialist bodies, and well-established partnerships across Europe, the UEMS represents around 1.6 million medical specialists.
One of the UEMS’ main activities is CME (Continuing Medical Education) and CPD (Continuing Professional Development) and, under the impulse of a growing shift from voluntary to mandatory CME-CPD in Europe, the European Union of Medical Specialists set up the European Accreditation Council for Continuing Medical Education (EACCME) in October 1999, finally bridging the gap with the United States, where an Accreditation Council for Continuing Medical Education had been established in 1981.
The EACCME – now a worldwide mark of excellence – sets itself as the central link between the National Accreditation Authorities (NAAs), the UEMS Specialist Sections and Boards (S&Bs), the European Specialty Accreditation Boards (ESABs) and the Providers of CME activities, accrediting more than 2,000 events per year. CME-CPD events in Europe are evaluated in ECMECs: 1 ECMEC = 1 hour of CME, with a maximum of 8 ECMECs for a day and 4 ECMECs for half a day.
In 2016, EACCME 2.0 addressed the new challenges of CME-CPD in Europe, from an ageing population to rapid technological developments, making the application/review/accreditation process smoother and expanding the portfolio of recognised activities and learning materials.
For the UEMS, CME-CPD is not only a clinical and professional duty, but overall an ethical obligation, for which health operators are life-long required to update their skills. CME-CPD should be free from commercial influence, meaningful during the entire professional life, affordable and – as far as possible – harmonised at national and European level.
In order to have a better framework on the current status of CME-CPD in Europe, in February 2019 the Section of Radiology has launched a survey on the current status of CME-CPD in Europe, targeting 31 member countries.
The information we searched for each country were:
1 Is CME-CPD mandatory or voluntary in your country? Number of credits required per year?
2 Are there any forms of re-certification or re-licensing?
3 Which is the responsible authority for CME-CPD?
4 Does a conversion scheme for accreditation of CME-CPD events abroad exist?
5 Which CME-CPD activities are recognized in your country?
6 Does your country follow the EACCME criteria?
7 Is CME-CPD onerous? How is it supported and promoted by the healthcare system?
The data for each country (30 NMAs have answered our survey) have been collected, analysed and summarised in some thematic maps below and in a table of results on the left column.
1 Is CME-CPD mandatory or voluntary in your country?
4 Does a conversion scheme for accreditation of CME-CPD events abroad exist?
6 Does your country follow the EACCME criteria?
7 Is CME-CPD onerous?
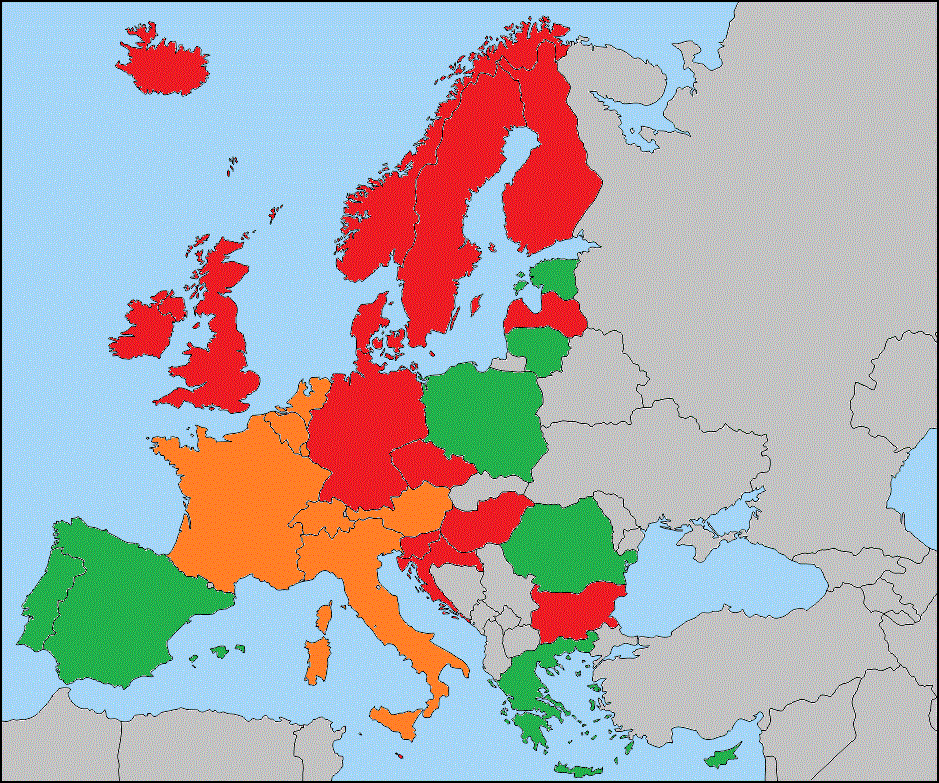
Yes
No
No answer
Partially/Not applicable
Yes
No
No answer
Partially/Not applicable
Yes
No
No answer
Partially/Not applicable
Yes
No
No answer
Partially/Not applicable
EACCME – European Accreditation Council for Continuing Medical Education
The UEMS established the European Accreditation Council for Continuing Medical Education (EACCME), in January 2000, with the aim of encouraging high standards in development, delivery and harmonization of continuing medical education. This was to be achieved through the international accreditation of CME events, and the establishment of a system for the international acceptance of CME points. Agreements in place with the American Medical Association (AMA) and the Royal College of Physicians and Surgeons of Canada (RCPSC) for mutual recognition of credits enhance the international status of EACCME. In April 2009 the UEMS-EACCME launched the accreditation of e-learning materials. By e-learning is meant the delivery of CME-CPD by methods including: recorded audio, recorded visual, recorded on Compact Disc (CD), recorded on Digital Versatile Disc (DVD), available on Personal Digital Assistant (PDA), available online via an educational website, or any mixture of the preceding. The UEMS-EACCME sets itself as the central link between the National Accreditation Authorities (NAAs), the UEMS Specialist Sections and Boards (S&Bs), the European Specialty Accreditation Boards (ESABs) and the Providers of CME activities.
EACCME 2.0
- Application-review-accreditation: faster and more efficient
- faster path for “Trusted Providers”
- Brand new IT platform
- Embrace state of the art educational events (e-platforms, e-libraries, apps)
- Accreditation portfolio: new forms of CME-CPD activities
- More emphasis on quality control of events
- Recognition of professional qualifications
- Embrace a broader spectrum of healthcare professionals
Towards EACCME 3.0!
Stay tuned!
For detailed information, please visit: https://www.uems.eu/uems-activities/accreditation/eaccme
ACI – Accreditation Council in Imaging
The European Board of Radiology is an organisation dedicated to the investigation, development and implementation of certification and accreditation activities and programmes, including examinations and other instruments of qualification/certification for general and sub-specialised physicians, programme evaluation and accreditation of continuing medical education activities, accreditation of training institutions and similar organisations, as well as monitoring and harmonising qualification and training standards in the field of radiology, imaging diagnosis in Europe. The specialist body of EBR, which is carrying out the proceeding of the accreditation in collaboration with the EACCME, is called Accreditation Council in Imaging (ACI).
ACI started its activities in January 2016 and now has a leading position in Europe and beyond. Since its creation, 732 Live Educational Events, 17 E-learning material and 2 packs of webinar applications have been received (as of January 31, 2021).
The Section is an important link in the fruitful collaboration between the EACCME and the ACI: official agreements have been signed in 2015 and amended in 2016 and 2017. Today, radiology – together with oncology and cardiology – is one of the UEMS specialties with the largest number of applicants for event accreditation.
As part of the agreement, UEMS and EBR representatives lead ACI on alternate basis (biannual mandate). Since 04 March 2019, Prof. Miloš Lučić is the new ACI Scientific Director. Representatives of the Section sit in the two ACI Scientific Committees. In particular: Prof. P. Ricci is the UEMS representative in Policy Committee, Prof. H. Aronen and Dr. R. Demuth in Reviewing Committee. According to ACI Rules of Procedure, mandate of ACI Committee members is now made biannual.
Since European accreditation systems differ not only from one another, but also with regard to their equivalence to ECMEC credits, in 2017 the ACI conducted the survey “Accreditation systems in Europe” in an attempt to collect relevant data on accreditation systems in Europe, and regarding significant preferences of the European radiological community on Continuing Medical Education (CME). Recognising the globally emerging generation shift not only in radiology, but also in the generation-dependent level of readiness to use current and future technologies in radiology and education, the ACI conducted a new survey in June 2018 on the future of Continuing Medical Education (CME) and Continuing Professional Development (CPD).
Given the fact that not only radiology, but our whole world, is becoming technology-oriented and technology-based, the ACI applied the generation scaling that is generally accepted in global social sciences terminology, dividing the generation distribution into various groups: the so-called Silent Generation/Traditionalists, born before 1946; Boomers/Baby Boomers, born between 1946 and 1964; Generation X, born between 1965 and 1980; Generation Y, also known as Millennials, born between 1981 and 1994 and Generation Z, born after 1994.
The overall results indicate that most respondents know how to earn CME credits (68.56%). However, it is evident that the best-informed radiology population are the older generation groups —Silent Generation/Traditionalists (80.95%), Boomers/Baby Boomers (78.73%) and Generation X (71.15%)— in comparison to Generation Y, with a much lower number of informed respondents (47.62%). It is also notable that in the same group the lack of knowledge about how to obtain CME credits, or the need for more information, is over 50% (52.38%). This unexpected fact is a cause for alarm, and should be understood as an immediate “call for action”, to inform younger generations of radiologists about how to earn CME credits.
With regard to CME credits obtained through attending Live Educational Events (LEEs) and E-Learning Materials (ELM), it appears that the great majority of radiologists and other radiology professionals, regardless of generation, are still earning the largest number of CME credits in a traditional way (LEEs), which includes congresses, seminars, courses and schools. We can see a slight shift among Generation Y respondents, together with a slight but observable increasing trend in earning more CME credits through ELM among the younger generation groups, indicating an easy and slow generational shift towards ELM.
ESOR – European School of Radiology
The European School of Radiology (ESOR) is an institution, fulfilling the mission of the European Society of Radiology (ESR) in the field of education. One of its main goals is to assist in harmonising radiological education in Europe. With its wide range of activities ESOR additionally aims to raise standards in the field of scientific radiology, to extend and coordinate teaching resources worldwide and to help young radiologists to achieve the knowledge and skills to fulfil tomorrow’s requirements.
ESOR consolidates position as global leader in radiology education with over 43 courses in 24 countries in 2020. A total of 124 scholarships and fellowships will be offered to trainees and young radiologists, enabling them to spend time in departments outside of their home country. The full list of training opportunities can be found here: https://www.esor.org/cms/website.php
Link: http://esor.org/cms/website.php
Eurorad
More than 7,500 published cases make EURORAD the largest peer-reviewed database for radiological case reports!
Eurorad – a project of the European Society of Radiology – is the largest peer-reviewed databse for radiological case reports, containing more than 7.000 published cases and is continuously growing. Celebrating its 20th anniversary, the brand-new website of Eurorad includes a fast and efficient search engine, Editors Selection of cases, teaching cases with quiz questions and much more.
Eurorad website (on the left column) is free to access.
ESR Connect
ESR Connect is the new video platform from the European Society of Radiology providing you with educational and entertaining videos in the field of radiology. Starting this fall, ESR Connect will offer exclusive live streaming events, an extended and constantly growing on-demand library and premium topic packages from the European Congress of Radiology.
ESR Connect comes up with 3 channels: Live, On-Demand and People.
- The Live Channel is where all future live events will be broadcasted (ESR Connect Weekly Live, ESOR Courses, ECR 2020,…).
- The On-Demand Channel includes recordings of the ESOR AI Course and Topic Packages from ECR 2019. More packages will continuously be added to this channel in future.
- The People Channel includes Interviews, Highlight Videos etc.
Choose your favourite content and watch it from wherever, whenever you want.
ESR Connect is accessible on the left column.
Survey Results
Survey: Interventional Neuroradiology in Europe
The current framework of Interventional and diagnostic Neuroradiology in Europe still presents significant differences in training and practice. In view of the preparation of the “European Training Requirements in Interventional Neuroradiology” – unanimously endorsed by the UEMS Council on April 13, 2019 – the Section of Radiology and the subspecialty Division of Neuroradiology have launched in September 2018 a survey on the situation of Neuroradiology training and Neuroendovascular practice in Europe. The survey has targeted radiology societies and national delegates from 31 UEMS member countries, belonging to the European Union, the European Economic Area and the Council of Europe.
The results of the inquiry have been summarised in the table and in the conceptual maps on the left column.
Edited by:
Dr. Francesca B. Pizzini – Secretary General, Division of Neuroradiology
Mr. Francesco Tanzi – Secretariat, Section of Radiology
Prof. Marek Sasiadek – President, Division of Neuroradiology
Prof. Paolo Ricci- President, Section of Radiology
Question 1 – Specialty school/Fellowship in Neuroradiology
□ Yes
□ No
□ N/A
□ Not answered
Question 4 – Neuroendovascular procedures performed by Interventional Radiologists
□ Yes
□ No
□ N/A
□ Not answered
Question 6 – INR Training required
□ Yes
□ No
□ N/A
□ Not answered
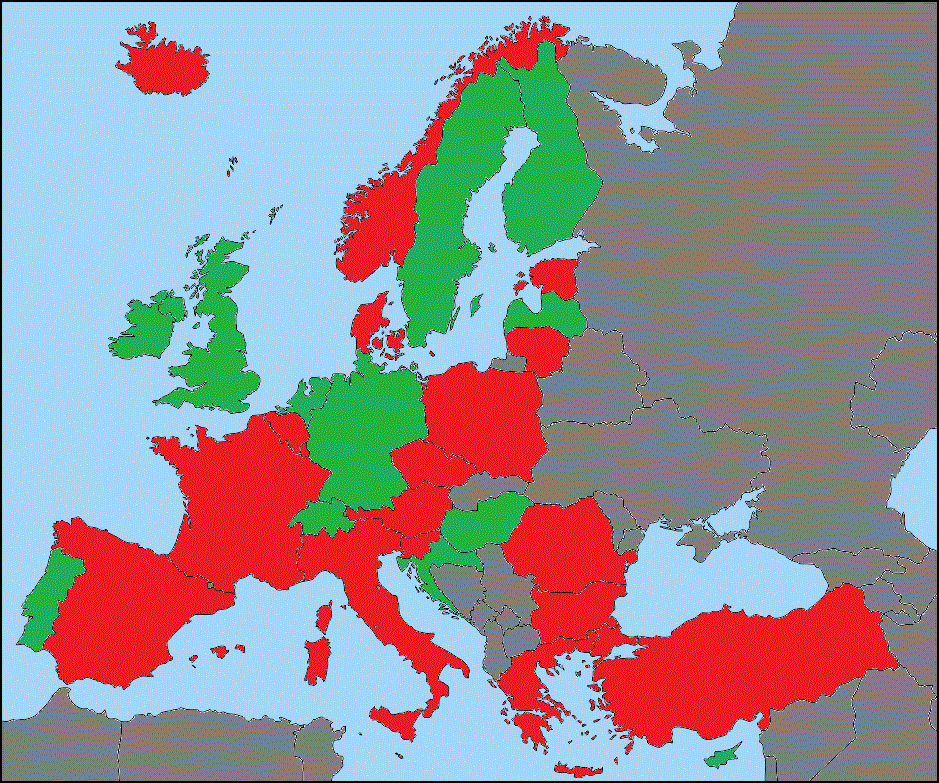
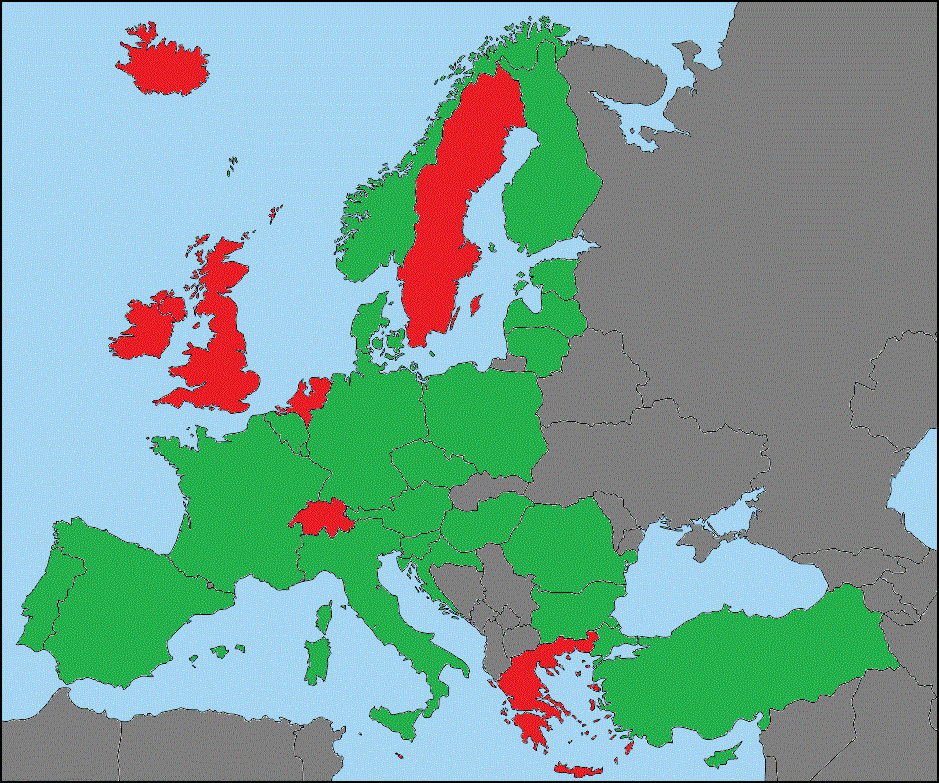
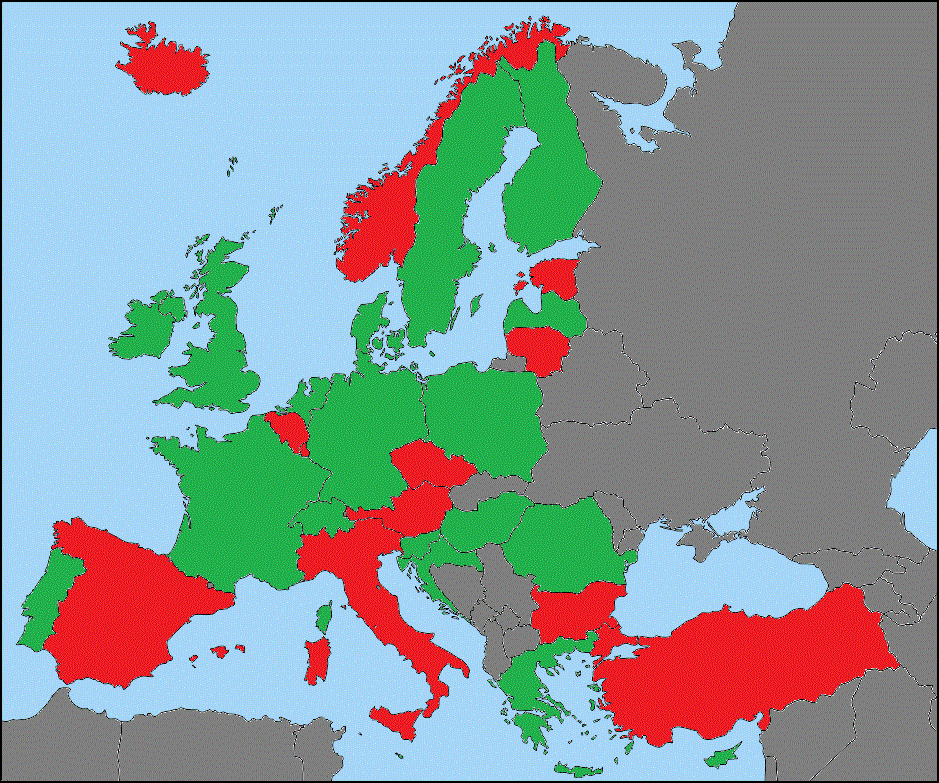
Radiology Workforce in Europe
A survey from the Section of Radiology
The Section of Radiology has several objectives, including the study, promotion and harmonisation of the highest level of training of the medical specialists, medical practice and health care within the European Union in Radiology; the study and promotion of free movement of radiologists within the EU, and the defense of the professional interests of European radiologists; the representation to EU authorities and any other authority and/or organisation dealing with questions directly or indirectly concerning the specialty of Radiology.
In order to better serve its mission, and actively collaborate with the most important scientific societies, professional and patient organisations and partners, in July 2019 the Section has addressed the UEMS National Member Associations of Europe to inquire about the most updated facts & figures on the Radiology Workforce in Europe. A repository of precious information has been created and will be constantly updated. You can consult the results on the left column.
Practice
General Data Protection Regulation
On January 25, 2012, the European Parliament and the European Council have adopted a first draft proposal for the regulation of sensitive personal data with particular regard to their processing, movement or distribution. This had been done to address the uncertainty of a very fragmented legislative framework between 28 Member States and since the last concerning Directive had been adopted in 1995 (Directive 95/46/EC).
In the field of health care, this first proposal has provoked a massive request from medical professionals’ organisations, patients´ associations and industry representatives for a more balanced approach to the topic, allowing access to health data and statistics for research purposes. After prolonged discussions, a revised agreement on data protection has been adopted by the European Parliament in April 2016 and officially published in the EU Official Journal on May 04, 2016. This Regulation (EU – 2016/679) will apply from May 25, 2018, meaning that Member States still have two years to transpose the provisions of the directive into their national law. It is an essential step to strengthen citizens’ fundamental rights in the digital age and facilitate business by simplifying rules for companies in the Digital Single Market;
Main outcomes are:
- Personal consent should be given by a clear affirmative act establishing a freely given, specific, informed and unambiguous indication of the data subject’s agreement to the processing of personal data, such as by a written statement or by electronic means.
- Processing of personal data for archiving purposes in the public interest, scientific or historical research or statistical purposes should be subject to appropriate safeguards for the rights and freedoms of the data.
- Right to data portability, including the health sector. Furthermore, the data subject will have the right to obtain any automated data which are processed using consent as the legal basis for processing in an easy-to-read format. This could mean hospitals and health and care providers being asked by patients to receive their electronic data in an appropriate format.
- Establishment of an independent supervisory authority for processing personal data at national/regional level which should be provided with the financial and human resources, premises and infrastructure necessary for the effective performance of the tasks.
- Genetic data should be defined as personal data relating to the genetic characteristics of a person which result from the analysis of a biological sample from the person in question, in particular chromosomal, deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) analysis.
- Data subject shall have the right to obtain from the controller the erasure of personal data concerning him/her without undue delay and any person has the right to have their personal data rectified or ‘the right to be forgotten’ but it still allowed the further retention of data when necessary in the public interest or in the exercise of official authority vested in the controller, for reasons of public interest in the area of public health, for archiving purposes in the public interest, or scientific and historical research purposes.
- Children and consent – parental prior consent is required for use of an under 13 year old’s personal data. Member States are free to set their own rules for those aged 13-15. If they choose not to, parental consent is required for children under 16.
- Data Protection Officer: organisations/companies above 250 employees must appoint a Data Protection Officer (DPO). This post can be shared with other organisations.
- National data protection authorities, also called supervisory authorities will continue to exist to protect fundamental rights in relation to data processing and to facilitate the free flow of personal data within the Union. Some countries (e.g. Germany) can keep more than one supervisory authority, but one of them has to be nominated as the representative on the new European Data Protection.
- Creation of the European Data Protection Board, which is established as a body of the Union with legal personality. The Board shall be composed of the head of one supervisory authority of each Member State and of the European Data Protection Supervisor and will ensure the consistent application of the Regulation, together with opinions, guidelines and best practices.
- Introduction of levels of fine up to 4% of the annual worldwide turnover for companies who misuse personal data, abuse the rights of the data subject or fail to implement requirements of the Regulation.
To summarise, the new regulation provides a more balanced approach between protecting privacy and making sensitive data available for research projects, where grounds of public interest (e.g. public health purposes) allow it. The processing of personal data concerning health may still be necessary for reasons of public interest in the areas of public health, without consent of the data subject. This in the consideration of the fact that sensitive data could be used to:
- better understand diseases and improve treatments
- understand patterns and trends in public health
- plan services that make the best of limited financial resources
- monitor the safety of drugs and treatments
- compare the quality of care provided in different states of the Union.
Once again, the new regime does not entirely rule out the relevance of national provisions. As stated in Recitals 8 and 10, in fact, the Regulation provides a margin of manoeuvre for Member States’ to restrict or specify its rules.
In addition to the EU legislative framework, the Organisation for Economic Cooperation and Development (OECD) establishes that “Informed consent has become the pillar for protecting individual’s autonomy where research involves human subjects.” In particular, the OECD data protection legislation grants patients (as data subjects) a number of rights once identifiable data are processed. The following are included:
- to be aware of the processing of their data, its purposes, the identity of the data controller, the identity of the possible recipients of the data;
- to have access to a copy of the information comprised in their personal data;
- to object to processing;
- to prevent processing for direct marketing;
- to object to decisions being taken by automated means;
- to have inaccurate personal data rectified, blocked, erased or destroyed;
- to claim compensation for damages caused by a breach of the Privacy Directive.
Member States are allowed to apply more stringent rules to legitimize the data subject’s consent, through the provision of written consent or preventing consent from being the sole basis to authorize the generally prohibited processing of personal health data. The data subject may always revoke the given consent at any time and without justification. This will impede further processing, but not making past operations retrospectively unlawful. Consent must be a “freely given, specific and informed indication of the data subject’s wishes”. The Privacy Directive does not require the consent to be in written form.
- Freely given consent: “Free” consent means a voluntary decision, by an individual in possession of all of his faculties, taken in the absence of coercion of any kind, be it social, financial, psychological or other. Furthermore, agreeing to undergo a certain medical treatment does not automatically furnish an explicit consent to the processing of personal data collected during such treatment.
- Specific consent: “Specific” consent must relate to a well-defined, concrete situation in which the processing of medical data is envisaged.
- Informed consent: “Informed” consent means consent by the data subject based upon an appreciation and understanding of the facts and implications of an action: The individual concerned must be given, in a clear and understandable manner, accurate and full information of all relevant issues (…) such as the nature of the data processed, purposes of the processing, the recipients of possible transfers, and the rights of the data subject. This includes also an awareness of the consequences of not consenting to the processing in question.
- Consent withdrawal: The consent could be considered as the weakest way to process data as the data subject has a total control on it without any consideration on the “scientific interest” for the whole society. The withdraw of consent may occur damage to the data controller as it can invalidate all the research in some cases even when the data subject has no real or valid reason.
Link to EC Data Protection Regulation: https://ec.europa.eu/info/law/law-topic/data-protection_en
How does every EU country processes Health Data afther GDPR? The EU-funded AEGLE project has prepared several updated country reports:
http://www.aegle-uhealth.eu/en/aegle-in-your-country/the-campaign.html
Since the new rules will come into force on May 25, 2018, the ESR – European Society of Radiology has prepared a paper on The EU General Data Protection Regulation: what the radiologist should know includeing an overview of the most relevant legislation applicable to radiologists, a glossary and concrete examples of the impact of GDPR on clinical practice.
Regulation of Professions
EU Proportionality Directive on the regulation of professions
On December 19 2011, the European Commission first published a proposal for a revision of the previous Professional Qualifications Directive based on the outcome of the various consultation processes. On October 9, the European Parliament adopted the proposed legislation, which was followed by the adoption of the agreed text by the Council on November 15, 2013.
The main elements of the directive were the following:
- Alert mechanism – Competent authorities of member states will have to proactively alert the authorities of other member states about professionals who are no longer entitled to practice their profession due to disciplinary actions or criminal conviction, through a specific alert mechanism.
- Language assessment – Competent authorities will be enabled to assess the language competence of professionals after recognition but before providing access to the profession.
- Continuous medical development and education for health professionals – Member states are to promote the continuous professional development of professionals who benefit from the automatic recognition of their professional qualification. This will apply to medical specialists, general practitioners, pharmacists and nurses.
- Harmonisation of minimum training requirements – Revised minimum training requirements for some of the healthcare professions, updating of the minimum training requirements for these sectors as well as a requirement for member states to encourage continuing professional education and training.
- Provision of CME and continuing training.
- European Professional Card (EPC) for interested professions: an ad-hoc focus group has already been established to implement the professional card
- No partial access for health professionals who benefit from automatic recognition, or for other professionals if there are public health or patient safety implications.
In 2016, regulated professions accounted for about 22% of the European labour force, representing around 50 million citizens. As part of the value chain and their role as input to production processes as well as their output, services provided by professionals, such as engineers, architects, accountants, lawyers, are crucial to the functioning of the European economy more broadly and across sectors. Excessive regulatory barriers to professional entry have negative consequences for job creation, productivity, mobility and the consumer who is forced to pay higher prices by a less competitive market.
Professional activities are regulated by Member States at national, regional or sometimes at local level. Securing the modernisation of the regulated professions and in particular the review of reserves of activities and the cumulative impact of measures requires that proper proportionality analysis be carried out at national level.
The first European Commission´s proposal for a Proportionality Directive in the regulation of professions was presented on January 2017, and since then the co-legislators have moved at very different speeds: while the Council completed its negotiations in the adoption of a general ap-proach in May 2017, the European Parliament is still in the early stages of adopting its position and a decision is expected for the autumn.
The right to work in the sense of the freedom to pursue one’s chosen profession or to conduct a business is enshrined in the Charter of Fundamental Rights of the EU. The key benefits of the EU Single Market include the freedom of establishment and the freedom to provide services.
The proposal will introduce a general obligation for member states to carry out an ex-ante proportionality assessment before adopting new or modifying existing legislation restricting access to or the pursuit of regulated professions. It must be also considered that economic considerations can only partially affect the health professions, as patient safety and access to healthcare are equally important.
The first deliberations of the European Parliament have proposed more extensive changes to the draft Directive, including an exemption for health professions as included in the draft Report of the Commit-tee for Internal Market and Consumer Protection (IMCO) and in the draft Opinion of the Committee for Envi-ronment, Health and Food Safety (ENVI). Several health organisations have raised their voice concerned by the potential impact of the Directive on their practices: as an example, CPME, alongside other health stakeholders, in particular the Council of European Dentists (CED) and the Pharmaceutical Group of the European Union (PGEU), have highlighted the fundamental difference of conceptual approach to regulation demonstrated by the draft Directive in comparison to the rationale for regulating doctors and other health professions.
On 4 December 2017 the European Parliament’s IMCO committee took the vote on its position on the Proportionality Directive. On the crucial issue of the exemption of health professions from the Directive, the proposal was defeated (by 20 votes to 15). The European Parliament will therefore now go into negotiations with the Council without a clear mandate to exempt health professions. A special status could in any case be granted health professions, which may help to mitigate the Directive’s impact.
Link: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2016:0822:FIN

Declaration of Geneva – The “Modern Hippocratic Oath”
The Declaration of Geneva is one of the World Medical Association’s (WMA) oldest policies adopted by the 2nd General Assembly in Geneva in 1947. It builds on the principles of the Hippocratic Oath, and is now known as its modern version.
The following Declaration has been adopted by the 2nd General Assembly of the World Medical Association (WMA) Geneva, September 1948 and amended by the 68th WMA General Assembly, Chicago, October 2017.
As a Member of the Medical Profession:
I SOLEMNLY PLEDGE to dedicate my life to the service of humanity;
THE HEALTH AND WELL-BEING OF MY PATIENT will be my first consideration;
I WILL RESPECT the autonomy and dignity of my patient;
I WILL MAINTAIN the utmost respect for human life;
I WILL NOT PERMIT considerations of age, disease or disability, creed, ethnic origin, gender, nationality, political affiliation, race, sexual orientation, social standing or any other factor to intervene between my duty and my patient;
I WILL RESPECT the secrets that are confided in me, even after the patient has died;
I WILL PRACTISE my profession with conscience and dignity and in accordance with good medical practice;
I WILL FOSTER the honour and noble traditions of the medical profession;
I WILL GIVE to my teachers, colleagues, and students the respect and gratitude that is their due;
I WILL SHARE my medical knowledge for the benefit of the patient and the advancement of healthcare;
I WILL ATTEND TO my own health, well-being, and abilities in order to provide care of the highest standard;
I WILL NOT USE my medical knowledge to violate human rights and civil liberties, even under threat;
I MAKE THESE PROMISES solemnly, freely, and upon my honour.
For further information: https://www.wma.net/policies-post/wma-declaration-of-geneva/

Gender equity – Women in Radiology
Although important measures have been taken and radiology seems to be one of the few medical disciplines in which female and male academic physicians make the same salaries, incentives to combat gender bias in the radiology profession are still needed to encourage diversity and the inclusion of women among researchers, according to a recent study published on June 13 in Academic Radiology. While gender disparities in clinical practice have been deeply investigated in the last few years, but disparities in radiology research and publications have not been tracked as closely, according to the US team of NYU Langone Medical Center, who conducted the study. To investigate challenges to radiology research and publication for women in radiology, they sent an electronic survey to 280 members of the American Association for Women Radiologists in September and October of 2017. The researchers received 89 surveys, for a response rate of 31.8%. Of the survey respondents, 61.4% indicated they were interested in conducting radiology research and 60.2% stated their departments expected them to do so. However, 80.7% of respondents also noted that their departments expected them to conduct educational activities.
The authors also found the following:
- 56.8% of survey respondents felt their research success was valued by their department.
- 47.7% felt they received appropriate credit for their research.
- 41.6% had ever had a female research mentor.
- 23.9% believed that their department makes deliberate efforts to support women’s research.
- 22.7% felt they received sufficient time for research.
Respondents listed family or child care issues, unconscious bias at the department or chair level, exclusion of women from research activities by male researchers, and concern about being perceived as “aggressive” as key barriers to conducting radiology research and stated that dedicated research time (40.4%), personal research mentors (23.6%), and earlier career training in research methodology (21.3%) were the most helpful factors for research success.
The findings suggest that a number of interventions are urgent to foster the research success of women radiologists, including dealing with conscious and unconscious bias among departmental and institutional leadership; establishing start-up and ongoing research time and access to department resources; putting work-life balance and child care policies in place; ensuring research tracks are available to both men and women in the department; and providing training in research methodology, negotiation skills, and leadership.
Closing the gender gap in Radiology – Lessons from ECR 2019
Women in Focus: Be Inspired was one of the most innovative programmes featured at the 2019 European Congress of Radiology in Vienna. Prominent female and male professionals from academic medicine, public health and the healthcare industry shared their views on topics relating to female leadership.
The publication below informs of the gender gap in the radiology profession, where the proportion of women still decreases at higher academic ranks and levels of leadership. The article reviews the current state of gender diversity in academic and leadership positions in radiology internationally and explores a wide range of potential reasons for gender disparities, including the lack of role models and mentorship, unconscious bias and generational changes in attitudes about the desirability of leadership positions.
Link:
A comparative study with Nuclear Medicine
In the US and Canada, men still outnumber women in academic performances and leadership positions of nuclear medicine by almost 6-to-1, according to a recent study published in the January issue of the American Journal of Roentgenology. In particular, researchers found that men occupy 86% of the leadership ranks despite the genders having comparable research experience.
These results show the need for devising strategies to promote diversity in leadership roles and tackle gender disparity in academic and leadership positions across nuclear medicine specialists.
| Nuclear medicine academic faculty members by gender (as of 2018): | ||
| Position | Male | Female |
| Professor | 35% | 16% |
| Assistant professor | 38% | 67% |
| Associate professor | 27% | 17% |

International Day of Radiology
On November 8, radiologists, radiographers, radiological technologists and professionals from related fields will celebrate the eighth International Day of Radiology all over the world. Let’s celebrate together!
The International Day of Radiology is an annual event held with the aim of building greater awareness of the value that radiology contributes to safe patient care, and improving understanding of the vital role radiologists and radiological technologists play in the healthcare continuum.
In 2020, the International Day of Radiology has been dedicated to all imaging professionals and their essential role in fighting the COVID-19 pandemic making an indispensable contribution to the diagnosis and treatment of COVID-19 patients.
Radiographers in Europe
ECR 2020 Radiographers Programme
As ECR is also the official annual scientific meeting of the European Federation of Radiographer Societies (EFRS), a five-day programme for radiographers will be made available at ECR 2020. Highlights will include Special Focus and Professional Challenges sessions, refresher courses, coffee and talks sessions and case-based diagnostic training.
Shape your Skills: Support Programme for Radiographers (Radiological Technologists) is financed exclusively by the ESR to support the continued professional development of radiographers. Successful applicants will be granted free congress registration and an accommodation voucher. Selection will be based on establishing a good geopolitical spread. Presenting authors of accepted abstracts will be preferred. Decision notifications will be sent out in December 2019.
Find complete information at: https://www.myesr.org/supportprogrammes
EFRS & ESR Joint Paper on patient safety in medical imaging
This joint paper of the European Federation of Radiographer Societies and European Society of Radiology highlights the diverse areas that encompass patient safety in medical imaging, and the important roles of both radiographers and radiologists in this. The paper can be accessed at the link below:
https://www.sciencedirect.com/science/article/pii/S1078817419300094
EFRS Diploma in Radiography (Radiotherapy)
Based on a partnership between the European Board of Radiology (EBR) and the European Federation of Radiographer Societies (EFRS), a new examination sitting for the EFRS Diploma in Radiography (Radiotherapy) has been scheduled for June 10 2019, in London. The examination is open to fully qualified radiographers who have completed their radiography education and training. Candidates must be officially recognised to practice in their country.
For further information and application:
ESR and ASRT to increase mutual collaboration on initiatives for radiographers
The European Society of Radiology (ESR) and the American Society of Radiologic Technologists (ASRT) have stepped up efforts to support radiographers together through a variety of different initiatives.
The ESR is increasing the membership benefits for North American radiologic technologists and offers radiographer a tailor-made Shape your Skills initiative for the continued professional development of radiographers. Successful applicants will be granted free ECR 2019 registration and a hotel accommodation voucher. On the other hand, ASRT has also committed to supporting a number of American radiographers attending ECR 2019 which is also recognised as the official scientific meeting in medical imaging for radiographers by both the ESR and the European Federation of Radiographer Societies (EFRS).
New at ECR 2019 – FIRST European Diploma in Radiography
Based on a cooperation between the EBR – European Board of Radiology and the European Federation of Radiographer Societies (EFRS), it will be possible for radiological technologists to sit a European Diploma in Radiography for the first time at ECR 2019! Two different exams will be available: an examination in medical imaging (incluidng nuclear medicine) or the combined examination for medical imaging (including nuclear medicine) and radiotherapy.
The first European Diploma in Radiography exam was held at the European Congress of Radiology 2019 on February 27 in Vienna. The examination is open to fully qualified radiographers who have completed their radiography education and training and are officially recognised to practice in their country.
More information on next events available at: https://www.myebr.org
Patient Safety
ESR Patient Advisory Group
The ESR Patient Advisory Group (ESR-PAG) was established in 2013 to bring together patients, the public and imaging professionals in order to collectively address and leverage developments in the field of radiology. The ESR-PAG advocates for a patient-centred approach in the activities of the ESR with the aim of benefiting patients across Europe. The ESR is the first medical specialty to successfully launch a professional-patient body that gathers patient representatives from various disease-specific fields and the ESR-PAG will become a group of committed patient advocates aimed at strongly embedding the patient perspective within radiology in full collaboration with the ESR.
For complete information about ESR-PAG, please visit:
https://www.myesr.org/quality-safety/patient-information
HPP Thematic Network on Medical Training and Professional Development for Patient Safety
On the EU Health Policy Platform, a new thematic network has been created by the European Society of Radiology (ESR) to focus on:
- how to promote evidence-based medicine through education;
- how education and mutual recognition of qualifications can enhance mobility of health professionals;
- the harmonisation of educational programmes and medical school curricula in the EU.
This thematic network seeks to explore the connection between education and professional development with enhanced patient safety. With the amount and quality of technological capabilities, scientific studies and available data increasing exponentially, it has become increasingly difficult for healthcare professionals to keep abreast of the latest developments, and to treat their patients according to the latest medical evidence. Creating a truly European healthcare labour force through university education and continuing medical education (CME-CPD) is therefore particularly important, yet many legal and political barriers remain for healthcare professionals that move to different countries.
In addition, the European Society of Radiology’s Patient Advisory Group (ESR-PAG) was established in 2013 to enhance the communication strategies with patient groups and organisations. The goal of the ESR-PAG is to bring together patients, the public, and imaging professionals in order to positively influence advances in the field of medical imaging to the benefit of patients in Europe. The last ECR 2017 in Vienna featured two well-attended sessions organised by the ESR-PAG which enjoyed high interest by the congress delegates and featured lively discussions between panellists and the audience.
Read more about ESR-PAG at: http://www.myesr.org/article/1067
Link to the EU Health Policy Platform: https://webgate.ec.europa.eu/hpf/
Radiation Protection
Joint IAEA and WHO Position Statement on the Bonn Call-for-Action
The International Conference on Radiation Protection in Medicine: Setting the Scene for the Next Decade held in 2012 in Bonn, Germany had the specific purpose of identifying urgent issues arising in medical radiation protection. Participants from 77 different countries attended the Conference, whose main outcome has certainly been a proposal on incoming priorities regarding radiation protection in the next decade. This specific outcome has been called Bonn Call for Action.
Its aims are:
a) to strengthen the radiation protection of patients and health workers overall;
b) to attain the highest benefit with the least possible risk to all patients by the safe and appropriate use of ionizing radiation in medicine;
c) to aid the full integration of radiation protection into health care systems;
d) to help improve the benefit/risk-dialogue with patients and the public;
e) to enhance the safety and quality of radiological procedures in medicine.
Safety Standards for Radiation Protection
The Council of the European Union established directive 2013/59/Euratom, which sets basic safety standards for protection against the dangers arising from exposure to ionizing radiation. Member States had time until the 6 February 2018 to complete the process of transposition into their national regulations. Radiologists must be aware of some key elements, including the directive’s requirement that high-dose procedures such as CT or interventional radiology must be closely monitored, especially for procedures on children. The Directive laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation introduced specific articles relating to two different areas:
- Justification of medical exposures with respect to new types of practices and the interrelation with bio-medical research and early detection of diseases (art. 55 (2))
- Accidental and unintended exposures of individuals subject to medical exposure focusing on specific requirements on notification and recording of significant events (art. 63 in conjunction with art. 96).
The directive clearly states any equipment used for interventional radiology and CT, as well as any new x-ray machine, must have a device or a feature informing the practitioner at the end of the procedure of relevant parameters for assessing the patient dose. The device or feature must also have the capacity to transfer this information to the record of the examination. Uniform basic safety standards are established for the protection of individuals subject to occupational exposures, besides the medical and public exposures against the dangers arising from ionising radiation.
Sources of ionising radiation can be processed radioactive materials, nuclear installations, natural radionuclides, x-ray machines and cosmic radiation. The Directive applies inter alia to the manufacture, production, processing, handling, disposal, use, storage, holding, transport of radioactive material and certain radiation emitting installations as well as the exposure of workers to radiation.
Link to EC Radiation Protection legislation: https://ec.europa.eu/energy/en/topics/nuclear-energy/radiation-protection
BSS Transposition Project
The BSS – Basic Safety Standards – Directive (Council Directive 2013/59/Euratom) modernises European legislation on radiation protection by taking account of the latest scientific and technological progress to consolidate the existing Euratom radiation protection work into one single piece of legislation, merging five previous directives and upgrading a recommendation to become legally binding.
The new Directive entails substantial innovative requirements in several different areas, as the protection of patients in medical applications, improving the protection of workers, and enhancing the protection of the public from natural radiation sources. After entry into force of the Directive 2013/59/Euratom on 6th February 2014, which consolidated many different sets of legislation, Member States will have time until 6th February 2018 to bring into force the laws, regulations and administrative provisions necessary to comply with this Directive.
Thus, the European Commission approved a 15-month tender project in May 2016 to monitor the transposition of the BSS Directive into Member States’ national legislation and to support its implementation. This project was awarded to a consortium headed by the European Federation of Organisations for Medical Physics (EFOMP). Other participating organisations are the European Society of Radiology (ESR) and the European Federation of Radiographer Societies (EFRS).
Led by the Radiation Protection Subcommittee, the ESR – European Society of Radiology developed a summary of the BSS directive that includes the essentials for diagnostic radiolog and organised a meeting in Brussels, hosted by the European Commission on 24-25 January 2017.
Radiation Protection initiatives
EuroSafe Imaging
EuroSafe Imaging is the European Society of Radiology’s flagship initiative to promote quality and safety in medical imaging. The mission of EuroSafe Imaging is to support and strengthen medical radiation protection across Europe following a holistic, inclusive approach. The European Society of Radiology (ESR) has taken part in a wide number of EC projects in the area of medical radiation protection in cooperation with the European Federation of Organisations for Medical Physics (EFOMP), European Federation of Radiographer Societies (EFRS), Heads of the European Radiological Protection Competent Authorities (HERCA), European Association of Nuclear Medicine (EANM), European Society for Radiotherapy & Oncology (ESTRO), and subspecialty radiological societies, such as the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) and the European Paediatric Radiology Society (ESPR). The ESR has also worked with major international organisations like the European Commission, International Atomic Energy Agency (IAEA), World Health Organisation (WHO), United Nations Scientific Commission on the Effects of Atomic Radiation (UNSCEAR) and the International Commission on Radiological Protection (ICRP).
At ECR 2019, the EuroSafe Imaging campaign has celebrated its fifth anniversary. To date, the ESR’s EuroSafe Imaging campaign has attracted more than 51,500 ‘Friends’ supporting its activities, including dozens of institutional supporters around the world. The first EuroSafe Imaging Call for Action was published in 2014. It was updated in 2018, becoming a 13-point action plan for achieving EuroSafe Imaging’s objectives of promoting appropriateness in radiological imaging, maintaining radiation doses within diagnostic reference levels, promoting the use of up-to-date equipment, empowering patients, joining forces with various stakeholders, and contributing to a global safety culture in medical imaging.
The EuroSafe Imaging Stars initiative was launched in 2016 with the goal of establishing a worldwide network of imaging departments committed to best practice in radiation protection. To participate in this initiative, imaging facilities have to perform a simple self assessment according to a list of 21 criteria on the topics optimisation, justification, quality and safety, education, research, and regulatory compliance. After successful evaluation, applicant facilities are awarded a number of stars from one to five, depending on the criteria fulfilled.
EUCLID – safety in medical imaging (2021)
The EUCLID (European Study on Clinical Diagnostic Reference Levels for X-ray Medical Imaging) project, a 33-month European Commission tender project run by the European Society of Radiology which began in August 2017, has come to a successful conclusion with the publication of European Commission Radiation Protection series publication 195: European study on clinical diagnostic reference levels for X-ray medical imaging. The EUCLID project established a network of hospitals covering 14 countries across Europe to work on the project and supply data on radiation doses.
More information here: http://www.eurosafeimaging.org
EURAMED
The European Alliance for Medical Radiation Protection Research (EURAMED) is a consortium of associations involved in the application of ionising radiation and radiation protection standards in medicine, namely the European Association of Nuclear Medicine (EANM), the European Federation of Organisations for Medical Physics (EFOMP), the European Federation of Radiographer Societies (EFRS), the European Society of Radiology (ESR) and the European Society for Radiotherapy and Oncology (ESTRO).
The first edition of a strategic research agenda (SRA) for medical radiation protection has been published in July 2016.
Artificial Intelligence, Machine Learning and Virtual Reality
In late 2016, AI luminary – Prof. Geoffrey Hinton – was famously quoted as saying it was obvious ‘we should stop training radiologists’ because they’ll soon be replaced by AI. Although specific tasks can be done with software, AI has not delivered on all its early promise, according to many observers, and it is not replacing radiologists. Some radiologists, however, argue AI is launching its assault on radiologists.
The term Artificial Intelligence (AI) is applied when a device mimics cognitive functions, such as learning and problem solving. It refers to a field of computer science dedicated to the creation of systems performing tasks that usually require human intelligence. Machine learning (ML), a term introduced by Arthur Samuel in 1959 to describe a subfield of AI including approaches that allow computers to learn from data without being explicitly programmed, has been extensively applied to medical imaging. Among the techniques that fall under the ML umbrella, Deep learning (DL) has emerged as one of the most promising.
You can find a comprehensive framework on AI and ML definitions and applicability in the interesting paper – Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine, appeared on European Radiology Experimental in 2018. Link: https://eurradiolexp.springeropen.com/articles/10.1186/s41747-018-0061-6
Although radiologists are not the only medical professionals to have their specialty modified by Artificial Intelligence, it is widely accepted that the radiological community is on the verge of a major revolution, and that AI will deeply affect the radiology specialty and the way clinical specialty and professional life have been conceived. While a good AI technology may potentially be helpful and valuable, bad or unethical use may be extremely dangerous, with proper regulation needed to ensure that software is used safely, ethically, and with appropriate protection of patient privacy. Patients, radiologists, and regulatory authorities must work together to prevent that from happening and a balance that provides security, privacy protection, and ethical use of sensitive information.
As Prof. H. Lamb – member of the Executive Bureau of the Section – thinks: “an important challenge for radiologists is to maintain a high level of quality, whereas the ever-increasing workload can be facilitated. To guarantee high quality radiological exams and reports, assistance from AI can be of great value. Pre-processing of radiological imaging and especially image quantification can help to substantially reduce time spent per read. We can thereby create more time for communicating results, for example, in multidisciplinary meetings and in direct contact with patients. Commercial imaging clinics show and explain results to their clients. In routine clinical settings, radiologists do not have enough time to communicate directly with patients. The application of AI may create more time to connect with patients, and present imaging results in an app, on a mobile phone, automatically” (ECR Today 2019, Artificial or intelligent radiologist?)
In the European Union (EU), the current regulatory framework for medical devices – including AI software – has been reformed by the new Medical Device Regulation (MDR) and the new In Vitro Diagnostic Medical Device Regulation (IVDR). The MDR will become effective on 26 May 2020 while the IVDR will apply as of 26 May 2022. The recent reform originated from the awareness that the existing directives, dating back to the 1990s, are no more appropriate to deal with new, evolving technologies. On the other hand, the rise of AI generates several concerns relating to the way sensitive data are collected by the devices: as an example, data are threatened by cyberattacks addressed to those same bodies which collected them. Similar threats led the EU to adopt the General Data Protection Regulation (GDPR), which went into effect 24 May 2018, and the Cybersecurity Directive, which took effect on 10 May 2018. The extended territorial scope and wider rights for data subjects in the GDPR make the regulation more suitable to regulate AI, while the Cybersecurity Directive delineates requirements for EU member states that aim to prevent cyberattacks and to limit their effects.
“Among other provisions, member states are required to ensure that operators of essential services take appropriate measures to prevent and minimize the impact of incidents and to preserve service continuity (Articles 14(2) and 16(2)), and to ensure that supervisory authorities are notified of incidents without undue delay (Articles 14(3) and 16(3),”
ESR raises its voice on AI and digital health in Brussels
The European Society of Radiology (ESR), represented by its President, Prof. Boris Brkljacic, participated in a series of successful meetings and events on December 10, 2019 in Brussels to strengthen the position of medical imaging on the European Union (EU) health policy agenda. The ESR participated in the event entitled “Europe’s Beating Cancer Plan – Better access to cancer care in Europe?” hosted by EU40, a group of young Members of the European Parliament under the age of 40. During the Q&A session, Prof. Brkljacic intervened by highlighting the contribution of radiology towards accurate diagnosis and treatment. Furthermore, he called on the EU institutions to act and to gradually implement lung cancer screening programmes following successful clinical trials that have demonstrated their effectiveness on the overall mortality rate.
In an interview with EurActiv, Prof. Brkljacic provided the society’s perspective on the digital transformation of healthcare and the uptake of Artificial Intelligence (AI) both in clinical practice and research. It was emphasised that imaging technologies were far ahead from other specialties in embracing AI, with the potential to generate rapid diagnoses as well as targeted and personalised treatments. Despite the potential for transforming healthcare systems, Prof. Brkljacic drew attention to ethical and privacy issues, which require for AI to be introduced alongside a clear framework guiding its development and use.
In addition, Prof. Brkljacic gave a voice to the medical profession’s views on digital health at the event “Digital Transformation of Healthcare – Unlocking the potential of digital solutions for patients, healthcare providers and health systems”, kindly hosted by GE Healthcare and the European Brain Council (EBC). The meeting brought together a panel of experts to discuss pressing issues on the path towards the digitisation of healthcare systems across Europe. Prof. Brkljacic underlined the radiology profession’s support for breakthrough innovation, which should be integrated into healthcare hand-in-hand with ethical and privacy standards, putting the patients’ interests first.
ESR and Major North American Organisations release a statement on AI Ethics in Radiology
In September 2019, major experts in the use of artificial intelligence (AI) in radiology, from many of the world’s leading radiology, medical physics and imaging informatics groups, published a statement to guide the development of AI in radiology. This multi-society statement focused on three major areas: data, algorithms and practice and sought to receive feedback from patients, radiologists, regulators and other stakeholders during a comment period that ended in April 2019.
The involved organisations included American College of Radiology, European Society of Radiology, Radiological Society of North America, Society for Imaging Informatics in Medicine, European Society of Medical Imaging Informatics, Canadian Association of Radiologists and American Association of Physicists in Medicine.
You can also find the statement published on Insights into Imaging:
https://insightsimaging.springeropen.com/articles/10.1186/s13244-019-0785-8
Risks and criticalities of AI:
Developers of AI for healthcare applications may have values that are not always aligned with the clinical values: there may be temptation, for example, to guide systems toward clinical actions that would improve quality metrics but not necessarily patient care. Or these algorithms may be able to skew data provided for public evaluation when being reviewed by potential hospital regulators. Furthermore, It is possible to program clinical decision-support systems in a manner that would generate increased profits for their designers or purchasers, such as by recommending tests, drugs, or devices in which they hold a stake, or by altering referral patterns.
Virtual Reality, on the contrary, has already a long history of applicability in radiology, although it is still far from mainstream market adoption. Anyway, the idea of performing advanced visualization and complex image analysis for diagnosis in an open 3D space, with all the tools and functionalities available at the touch of a virtual button or voice commands, seem incredibly attractive to physicians today, much more than two decades ago, when Virtual Reality was implemented. With basic technology requirements far more accessible today in terms of costs, use of Virtual Reality (VR) and Augmented Reality (AR) in healthcare and clinical settings is starting to gain traction. However, other barriers must be overcome: for instance, policymakers and government agencies need to define the ground rules for clinical use and reimbursement; and end-users of the technology need to work closely with the industry to help develop it to fit the needs and customs of real-world clinics. As an intermediate step, AR for surgical applications and image-guided therapy, which layers 3D images from scanners, segmentations, and measurements on top of the patient directly in the interventional suite, could be adopted in the mainstream. Despite VR being very interesting for healthcare applications, quite a few hurdles need to be overcome from the business point of view, as reduceing the psychological barrier by increasing exposure in and around the healthcare segment, increasing the user-friendliness of VR and AR applications, and developing them in association with physicians and radiologist to directly target their needs and reduce the implementation burden and giving effective proofs VR and AR can help save time and money and increase accuracy in diagnosis.
Assessing the impact of AI in radiology management:
“As the demand for time-consuming imaging services keeps on growing, radiology departments are increasingly vulnerable to staff shortages. Artificial intelligence (AI) can be a solution in many management scenarios, but leaders must address the enduring apprehension and define which tools are relevant when making the most of the new technology.”
Please find below the link to a very interesting interview on this topic with Prof. Christoph D. Becker collected at the ESR AI Premium Event in Barcelona (April 2019).
Link: https://ai.myesr.org/education/in-work-assessing-the-impact-of-ai-in-radiology-management/
An extraordinary competition between physicians and AI:
On June 30, 2018 in Beijing, an extraordinary radiology competition took place. It was heralded by the CHAIN Cup, an event billed as “The World’s First Competition between Physicians and Artificial Intelligence in Neuroimaging”. A custom-built Artificial Intelligence (AI) system dubbed BioMind was pitted against a team of 15 specialist doctors, with experience in neuroimaging. The AI system, dubbed BioMind, was programmed to accurately diagnose brain tumours and predict hematoma expansion. ESR Past-President – Prof. Paul M. Parizel – was one of the two international jury members.
AI won, by a large margin, predicting the correct histological diagnosis in 196 out of 225 cases (87%) in just under 15 minutes, whereas the human specialist doctors got only 66% right in 30 minutes. In the second heat, a different team of doctors competed with BioMind in predicting expansion of intracerebral hematoma; and AI won again by a large margin (83% versus 63%).
It must be highlighted however that – when checking the answers – the humans seemed more apt to provide a specific diagnosis, but they were more frequently wrong especially for ‘rare’ tumours. In any case, the AI BioMind system was not only accurate but also very fast. It was able to finish the 225 cases in 15 minutes (averaging 4 seconds per case), whereas the team of 15 specialised radiologists was able to achieve an accuracy rate of 66% when diagnosing 15 brain tumours each, finishing the task in 30 minutes (2 minutes per case).
ESR event “Intelligence. Innovation. Imaging – The perfect vision of AI” highlighted the importance of AI:
With more than 5,800 registrations from 140 countries for live streaming and 230 attendees onsite, the European Society of Radiology’s (ESR) event, ‘Intelligence. Innovation. Imaging – The perfect vision of AI’, which took place in Barcelona on April 05-06, 2019, highlighted the importance that artificial intelligence (AI) is playing for radiologists around the world.
In particular, the ethical use of artificial intelligence (AI) in radiology has been explored in the course of event by Dr. Adrian Brady, who also gave a speech on privacy, data use agreements and data protection. “Data use agreements should also be updated regularly to reflect new uses as well as version control specifications, he said. It’s probably true, however, that exclusive-use data use agreements are contrary to the common good, as they may prevent a significant amount of useful patient radiology data from being used to develop other AI tools or utilized in other research”, Dr. Brady said.
For further information:
ESR-UEMS Joint Session
The ESR-UEMS Joint Session is a special meeting annually held in Vienna, on the occasion of the European Congress of Radiology.
The Joint Session is the occasion for ESR and UEMS leaderships to present the many similarities and common strategies of the two organisations, in particular in the field of the defence of professional interests of radiologists and in the harmonisation of high-level radiology training in Europe.
The latest edition of the Joint Session has been dedicated to the topic: “Imaging Professionals within the EU. Radiologists without borders” and was focused, among other things, on the concept and importance of advocacy at the level of the European Union. The two societies have worked closely together for a number of years now and strive to further strengthen their cooperation
The whole session has been recorded and available, after registration, on ESR website:
An interesting article – appeared on AuntMinnie – has been dedicated to the ESR-UEMS Joint Session – 2019:
ESR-UEMS Joint Session 20190320
The ESR-UEMS Joint Session 2020 will be dedicated to the “Visibility of imaging professionals in the EU” and will take place on November 10-11 during the Online Highlight Week #9. Programme available on the left.
Save the date
UEMS Spring Council: online, April 23-24, 2021
UEMS CESMA meeting: online, June 19, 2021
UEMS Fall Council: Limassol, October 22-23, 2021 to be confirmed
